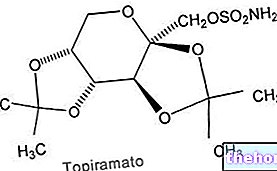
Topiramate is an anticonvulsant drug approved for the treatment of epilepsy and in the prophylaxis of some forms of migraine. It is a neuromodulatory drug, whose anticonvulsant activity is presumably due to the "inhibition of voltage-dependent sodium channels, to the" increase currents evoked by GABA, inhibition of currents evoked by kainate, inhibition of high voltage channels activated by calcium, and inhibition of carbonic anhydrase.

The "exact mechanism of action" by which topiramate helps weight loss has not yet been delineated; in any case, various mechanisms have been hypothesized: 1) increase in energy expenditure in the face of a reduced caloric intake linked to the anorectic effect; 2) reduction in the activity of salivary enzymes, important for the perception of food flavor; 3) reduction of leptin and corticosteroid concentrations; 4) reduction of glycaemia and insulinemia.
Topiramate has been shown to be effective for slimming purposes also in monotherapy, as evidenced by the results of some clinical studies conducted on patients suffering from obesity not related to complications, on patients with weight gain induced by psychotropic drugs, and on patients suffering from the syndrome of binge eating disorder. The most commonly reported adverse effects in these studies include paraesthesia, memory disturbances, taste distortion, fatigue, sleepiness, insomnia, difficulty concentrating and dizziness. To reduce these side effects, attempts have been made to experiment with new formulations of slow-release topiramate, which however do not appear to provide significant benefits. Generally, topiramate monotherapy is well tolerated, and although paraesthesias are common during use, their intensity is usually mild or moderate, rarely of such intensity that discontinuation of therapy is required.
The doses of topiramate monotherapy tested in the aforementioned clinical trials in the treatment of obesity ranged from 32 to 384 mg per day, in the context of a broader therapy involving behavioral modifications for weight loss, including the adoption of a low-calorie diet; these doses ensured on average a 5-15% greater weight loss compared to the control group. The most effective dose of topiramate in the treatment of obesity appears to be between 100 and 200 mg per day. Although the short- and long-term efficacy is comparable to that of sibutramine and orlistat, further studies are needed to better define the safety profile of the treatment.
See also: Topiramate for Quitting Smoking
Other articles on "Topiramate for Weight Loss"
- Phentermine
- Obesity - Drugs to Treat Obesity
- Anorectics - Anorectic Drugs and Supplements
- Orlistat
- Sibutramine