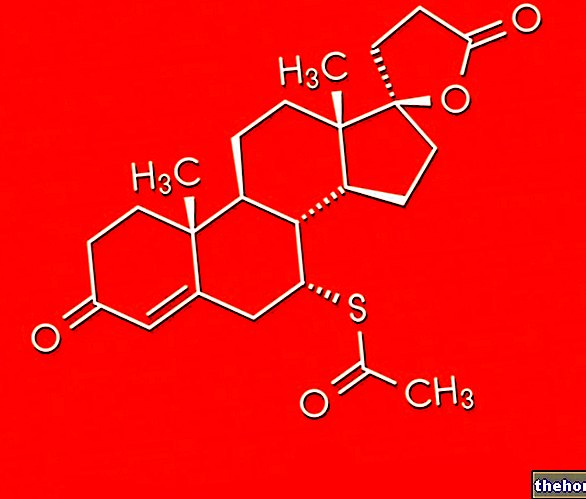
DIAMOX ® is an acetazolamide-based drug
THERAPEUTIC GROUP: Diuretics / Diuretics inhibitors of carbonic anhydrase

Indications DIAMOX ® Acetazolamide
DIAMOX ® is indicated in the treatment of edematous condition due to heart failure, with particular efficacy in improving pulmonary edema and relative dyspnoea in case of left heart failure. DIAMOX ® has also proved useful in reducing intraocular pressure typical of glaucoma and in assist with antiepileptic therapy, for which particular improvements have been observed especially for younger patients with less aggressive forms.
Mechanism of action DIAMOX ® Acetazolamide
DIAMOX ® is taken orally, and rapidly absorbed in the stomach. Maximum levels of its active ingredient, acetazolamide, are observed approximately 2 hours after oral administration, while urinary excretion becomes particularly consistent between the eighth and the twelfth hour.
Before being excreted intact with the urine, acetazolamide acts at the level of the proximal convoluted tubule of the nephrons, inhibiting an enzyme expressed by these cells, known as carbonic anhydrase. This enzyme catalyzes a reversible reaction which is very important for maintenance. of the acid-base balance and for the control of sodium reabsorption, which allows to hydrate carbon dioxide to carbonic acid (CO2 + H20 → H2CO3). The carbonic acid thus formed can dissociate into bicarbonate and proton ion, which it will be exchanged with sodium ions, which can thus be effectively reabsorbed.
The inhibitory action of DIAMOX on carbonic anhydrase therefore indirectly prevents a reabsorption of bicarbonate and sodium, increasing urinary excretion and diuresis. As an associated effect, there is an inevitable alkalinization of the urine and a slight haematic acidosis.
Studies carried out and clinical efficacy
1. ACETAZOLAMIDE AND MOUNTAIN SICKNESS
Wilderness Environ Med. 2010 Sep; 21: 236-43. Epub 2010 Jun 16.
Prospective, double-blind, randomized, placebo-controlled comparison of acetazolamide versus ibuprofen for prophylaxis against high altitude headache: the Headache Evaluation at Altitude Trial (HEAT).
Gertsch JH, Lipman GS, Holck PS, Merritt A, Mulcahy A, Fisher RS, Basnyat B, Allison E, Hanzelka K, Hazan A, Meyers Z, Odegaard J, Pook B, Thompson M, Slomovic B, Wahlberg H, Wilshaw V , Weiss EA, Zafren K.
Acute mountain sickness is a very frequent condition among trekkers, due to a difficult acclimatization dictated by the gradual and constant reduction of oxygen levels, which manifests itself with complex symptoms including headache. Acetazolamide at this juncture is particularly important, as by inducing a slight blood acidification it stimulates the respiratory system to a more effective gas exchange. This study conducted on 143 trekkers at about 4000 meters high, has shown how 85mg of acetazolamide can have the same effect on headache improvement as 600mg of ibuprofen, also presenting a preventive protective effect against pulmonary and cerebral edema.
2. ACETAZOLAMIDE AND FUTURE PERSPECTIVES FOR GLAUCOMA
Expert Opin Drug Deliv. 2010 Aug; 7: 943-53.
Promising complexes of acetazolamide for topical ocular administration.
Granero GE, Longhi MR.
Administration of acetazolamide is particularly important for the treatment of optic glaucoma, due to its ability to reduce intraocular pressure. However, the oral administration of this drug subjects the patient suffering from glaucoma to a series of side effects that significantly reduce the relationship between benefits and costs. For this reason, and given the efficacy of acetazolamide therapy, new administration protocols are being developed which provide for the typical ocular intake of the aforementioned drug and reduce systemic side effects.
3. ACETAZOLAMIDE IN PEDIATRIC USE
Can J Ophthalmol. 2010 Feb; 45: 41-5.
The effect of oral acetazolamide on weight gain in children.
Sharan S, Dupuis A, Hébert D, Levin AV.
This important study shows one of the most important limitations of acetazolamide treatment in pediatric glaucoma. More precisely - despite the drug's effectiveness in improving the pathology and the very low invasiveness compared to surgery - the most important limit to its spread in the pediatric environment is given by the induced growth retardation. In fact, during the therapy there is a low weight gain probably associated with the acidosis caused by the drug. However, to fully understand the complexity of reactions that develop in this period, it would be necessary to monitor other important parameters for the child's growth. .
Method of use and dosage
DIAMOX ® 250 mg tablets of acetazolamide / 500 mg capsules of acetazolamide:
- For the control of edema, about 1 and a half tablets per day are suggested, preferably taken in the morning.
- For the treatment of glaucoma, however, 2 to 3 capsules a day are recommended, or a tablet taken at 4/6 hour intervals.
- For the treatment of epilepsy, in the case of adjuvant therapy, 1 tablet per day is generally used; a dose that can increase according to the combined therapy and the patient's situation.
In any case, the correct dosage should be formulated by the doctor, after a "careful evaluation of the physio-pathological conditions of the patient.
IN ANY CASE, BEFORE TAKING DIAMOX ® Acetazolamide - YOU NEED THE PRESCRIPTION AND CONTROL OF YOUR DOCTOR.
Warnings DIAMOX ® Acetazolamide
The diuretic and saluretic action of DIAMOX ® requires constant monitoring of certain blood parameters such as: blood count, sodium, potassium, pH and even glycaemia in patients with diabetes mellitus, both before and during administration. It is also necessary to consider that the treatment with acetazolamide, especially if prolonged over time or incorrectly dosed, could cause an "alteration of the electrolyte and acid-base balance, with possible hyponatremia, hypokalaemia and metabolic acidosis."
DIAMOX ® must therefore be used with particular care in the case of patients with chronic obstructive pulmonary disease and emphysema, as a possible acidosis could contribute to the onset of tachypnea, anorexia, somnolence, lethargy and in the most serious cases also to coma.
Acetazolamide, structurally included among the sulfonamides, could be responsible for toxic epidermal necrolysis, aplastic anemia, agranulocytosis and other anaphylactic reactions in patients hypersensitive to these compounds.
The administration of DIAMOX ® could cause drowsiness, confusion or in any case alter the patient's normal perceptive and reactive skills, altering the ability to drive and use machinery.
PREGNANCY AND BREASTFEEDING
Experimental data regarding the use of DIAMOX ® and more generally of acetazolamide in pregnancy, suggest avoiding its use during the first trimester, subsequently limiting its intake only in case of unavoidable need. In fact, while studies conducted on humans do not show embryotoxic or teratogenic effects at doses of 250 mg / day, those conducted on laboratory animals have resulted in newborn animals with serious functional impairment and limb deformities.
Acetazolamide is also secreted intact in breast milk, albeit in small part; therefore it is recommended to stop breastfeeding during the administration of DIAMOX ®.
Interactions
DIAMOX ® can alter the functionality of various drugs such as:
- Aspirin and lithium salts, resulting in an "increased elimination, consequently a reduction in therapeutic effects;
- Amphetamines and tricyclic antidepressants, increasing their functionality following a reduction in their excretion;
- Mercurial and other diuretics, with a consequent increase in diuresis;
- Phenytoin (antiepileptic drug), increasing its plasma levels;
- Ciclosporin, with an increase in plasma levels.
- Antidiabetics, resulting in an alteration of the glycemic profile.
Furthermore, acetazolamide can cause an increase in urine crystals and kidney stones in conjunction with sodium bicarbonate therapy.
Contraindications DIAMOX ® Acetazolamide
Given the biological action of DIAMOX ®, its intake is not recommended in case of acidosis, alteration of the electrolyte balance (hyponatremia and hypokalaemia), hepatic, renal or adrenal insufficiency, and in case of hypersensitivity to one of its components.
Undesirable Effects - Side Effects
Most of the side effects of DIAMOX ® are observed in the initial phase of therapy and include:
- Paresthesia, tingling, decreased appetite, impaired hearing, nausea, vomiting, diarrhea, polyuria and in the most severe cases even drowsiness and confusion.
Prolonged therapy with DIAMOX ® can cause an "alteration of the electrolyte and acid-base balance", which can be remedied by administering bicarbonate, which however exposes the patient to a greater risk of kidney stones.
Haematological effects were also observed, characterized by agranulocytosis, leukopenia, thrombocytopenia, hypoglycemia, hyponatremia and hypokalaemia.
To the side effects listed above it is then necessary to add all those due to hypersensitivity towards acetazolamide, such as: skin rash, fever, anaphylactic reactions, erythema, myelosuppression and associated syndromes.
Note
DIAMOX ® can only be sold under medical prescription.
The use of DIAMOX ® should always take place after consulting your doctor.
The indiscriminate use of DIAMOX ® among athletes and non-athletes, to search for the loss of a few kilos, exposes the body to serious side effects. Furthermore, it is always advisable to reiterate that weight loss is dictated by the elimination of liquids and salts and not by a real slimming effect, intended as a loss of fat mass.
Therefore DIAMOX ® is classified among the DOPING substances.
The information on DIAMOX ® Acetazolamide published on this page may be out of date or incomplete. For a correct use of this information, see the Disclaimer and useful information page.