Generality
Alkylating agents are a class of drugs used to treat cancer. These molecules act by intercalating (ie inserting) alkyl groups between the two strands that make up the double helix of DNA.

In healthy cells there are defense mechanisms to repair damage that can occur to DNA. In tumor cells, on the other hand, these mechanisms are much less efficient and that is why diseased cells are particularly sensitive to the damage caused by alkylating agents. However, these compounds also show a certain toxicity towards healthy cells, especially at the level of those tissues that are characterized by rapid cell turnover, as occurs, for example, in the mucous membranes of the gastro-intestinal tract, in the bone marrow or on the skin. scalp.

DNA is made up of two strands joined around each other to form a double helix.
DNA is made up of many monomers, called nucleotides. There are 4 types of nucleotides: adenine (A), guanine (G), cytosine (C) and thymine (T), which combine with unique AT (adenine-thymine) and CG (cytosine-guanine) pairs held together by hydrogen bonds .
The sequence of bases present along the DNA molecule carries the genetic information.
Alkylating agents are dose-dependent, i.e. the amount of cancer cells that die is directly proportional to the amount of drug used.
They can be administered alone or in combination with other drugs and / or other therapeutic strategies.
Recently, it was discovered that the "hyperthermia, in combination with therapy with alkylating agents, is able to enhance its effects.
History
Prior to their use as antineoplastic chemotherapy, alkylating agents were better known as "sulfur mustards". Sulfur mustards are gods blistering gas (i.e. they create blisters on the skin) which were used as chemical weapons during the First World War.
Two pharmacologists - Louis Goodman and Alfred Gilman - began studying these compounds in 1942, at the request of the United States Department of Defense. The two pharmacologists observed that sulfur mustards were too volatile substances to be used in laboratory studies, so they replaced the sulfur atom (S) of the sulfur mustards with a nitrogen atom (N). nitrogenous mustards, characterized by lower volatility and greater stability.
Nitrogen mustards were the first alkylating agents to be studied for possible use in the treatment of tumors.
Types of alkylating agents
The alkylating agents used in the treatment of cancer can be divided into three categories, according to the way in which they perform their action.
Classical alkylating agents
The classical alkylating agents are defined as such because, in their structure, they present real alkylating groups which are inserted inside the double DNA strand. The alkylating group is bound to a nitrogen atom present in the guanine structure (one of the four nucleotides that make up the DNA).
This category includes:
- The nitrogenous mustards, among which stand out mechlorethamine, melphalan, chlorambucil, estramustine, cyclophosphamide, ifosfamide And uramustine.
- The nitrosoureas, of which they are part carmustine, lomustine And streptozocin.
- The alkyl sulfonates, among which we find the busulfan.
- The aziridine, among which we find the thiotepa (or tio-TEPA) and its derivatives. These drugs are usually considered classical alkylating agents, but can sometimes be considered as unconventional alkylating agents.
Compounds that act like alkylating agents
These compounds do not intercalate a true alkyl group in the double strand of DNA, but they bind to it in the same way that classical alkylating agents do.
This category includes i platinum organ complexes. Among these we find cisplatin, carboplatin, oxalylplatin And satraplatin.
Unconventional alkylating agents
These agents intercalate an alkyl group inside the DNA double helix, but - unlike the classical alkylating agents - the group is bound to an oxygen atom present in the guanine structure. This category includes the procarbazine and i triazeni (including decarbazine, mitozolomide And temozolomide).
Applications
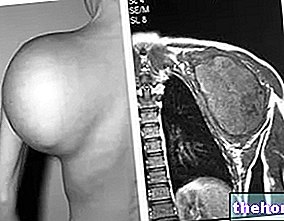
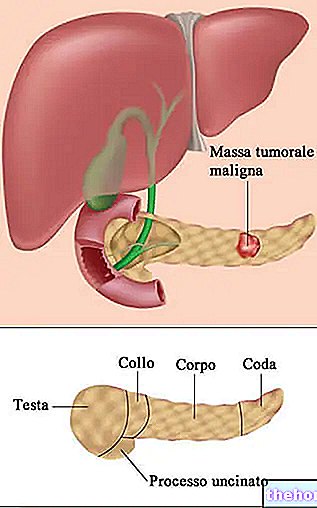
Alkylating agents are widely used in the treatment of numerous cancers, including leukemias, lymphomas, carcinomas and sarcomas. Some types of alkylating agents appear to be selective for specific tumors. Here are some examples:
- The nitrosoureas they are mainly used for the treatment of brain tumors;
- The melphalan it is used in multiple myeloma;
- The alkyl sulfonates they are used for the treatment of chronic myeloid leukemia;
- There thiotepa it is used for the treatment of breast and ovarian cancer and for papillary bladder cancer.