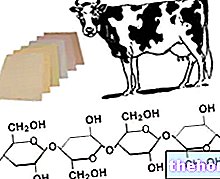
CELLULOSE is a homogeneous polysaccharide, which differs from starch in that it is formed by b-glucose, where the single molecules, with a B-1,4 bond, are rotated with respect to each other by 180 °; the fact that each molecule is rotated by 180 ° with respect to the one with which it is linked, causes the cellulose molecule to assume a linear structure; this rotation does not occur in the case of the 1,4 bonds of starch, and this is the reason therefore amylose, consisting of a-1,4 bonds of a-glucose, has a non-linear but spiral-shaped structure.

Cellulose can also be used in the production of carboxy-methyl-cellulose, a substance of herbal and cosmetic interest; it is considered a bulk, volume laxative to be taken together with high amounts of water.
Cellulose is also used in the production of explosives and various products of health interest. It is easily obtained from the common sources of fibers, from which products for textile use, or for medical-surgical aids (gauze, cotton wool) are also obtained. The source, in this case, is cotton, Gossipium irsutum; the drug consists of the protective hairs that surround the seed; hairs that are collected, processed and spun to obtain medical-surgical elements, which can also be sold in typical exercises in the sphere of herbal interest.
The microcrystalline cellulose is obtained instead from the waste of the wood processing, through a chemical - physical process called "wood explosion"; this process is carried out by placing such waste in an alkaline solution, at temperatures of 200 - 220 ° C and at pressures over 40atm; this favors the solubilization of the lignin, which passes into solution, while the subsequent and sudden passage from the pressure of + 40atm to the atmospheric one favors the disintegration of the cellulose fibers, which thus remain free in solution, to then be extracted with suitable reagents to obtain microcrystalline cellulose, useful as a film-forming substance, for the production of thickeners or excipients.
Other articles on "Cellulose Properties"
- Starch - starchy drugs
- Pharmacognosy
- Fructans, inulins and mucilages