.jpg)
What is Alli?
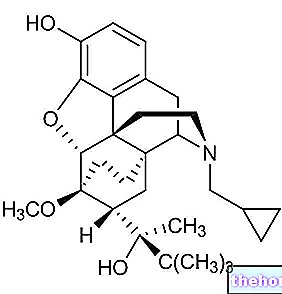
Alli is a medicine that contains the active substance orlistat, available as turquoise capsules (60 mg).
What is Alli used for?
Alli helps you lose weight. The medicine is used in overweight adults with a body mass index (BMI) greater than or equal to 28 kg per square meter, in combination with a low-calorie, low-fat diet.
The medicine can be obtained without a prescription.
How is Alli used?
Alli is given in doses of one capsule to be taken with water immediately before, during or within one hour of each main meal, three times a day. the drug The patient should follow a diet in which about 30% of the calories come from fat The food provided by the diet should be distributed between three main meals.
Patients intending to take Alli should start a diet and exercise regimen before starting treatment with the medicine. If weight loss does not occur after 12 weeks of treatment with Alli, you should contact your doctor or pharmacist.
You may need to stop taking it.
How does Alli work?
The active substance in Alli, orlistat, is an anti-obesity medicine that does not affect appetite. Orlistat is an inhibitor of gastrointestinal lipases (enzymes that metabolize fat).
The inhibition of these enzymes prevents the metabolism of some fats present in the diet, which allows about a quarter of the fats ingested during meals to pass through the intestine without being assimilated. The body cannot use these dietary lipids to produce energy or store them in fatty tissues. This promotes weight loss.
How has Alli been studied?
Since Alli is based on another medicine containing the same active substance already authorized in the EU (Xenical, 120 mg capsules), some studies involved patients treated with Xenical.
Alli has been studied in three main studies. Two of the studies involved a total of 1 353 overweight or obese subjects with a BMI of 28 kg / m2 or more and lasted from one to two years, comparing Alli at different doses with placebo (a dummy treatment), in association with a diet. Both patients and doctors only knew which treatment was given at the end of the study. The third study compared Alli with placebo and involved 391 overweight patients with a BMI between 25 and 28 kg / m2. The study lasted four months.
For all studies, the efficacy index was based on the change in body weight.
What benefit has Alli shown during the studies?
Alli was more effective than placebo in causing weight loss in subjects with a BMI of 28 kg / m2 or higher. In the two studies of patients with a BMI of 28 kg / m2 or higher, patients who received Alli 60 mg lost an average of 4.8 kg after one year, compared with 2.3 kg lost with placebo treatment. The study with Alli in subjects with BMI between 25 and 28 kg / m2 did not show significant weight loss for the patients.
What is the risk associated with Alli?
Most of the side effects associated with Alli affect the digestive system and are unlikely to occur in conjunction with a low-fat diet. Generally, these are mild symptoms that occur at the start of treatment and disappear after some time. The most common side effects with Alli (in more than 1 in 10 patients) are oily discharge, flatulence combined with stool emission, strong stimulation defecation, greasy / oily stools, oily bowel movements, flatulence and soft stools. For the full list of side effects reported with Alli, see the package leaflet.
Alli must not be used in patients who may be hypersensitive (allergic) to orlistat or any of the other ingredients. It must also not be used in patients treated with ciclosporin (used to prevent organ rejection in transplant patients) or with medicines used to prevent blood clots, including warfarin. It must also not be given to people with chronic malabsorption (a disease in which the nutrients in food are not easily absorbed during digestion) or from cholestasis (a disorder of the liver) or during pregnancy or breastfeeding.
Why has Alli been approved?
The Committee for Medicinal Products for Human Use (CHMP) considered that Alli's benefits are greater than
risks for facilitating weight loss in overweight adults (BMI ≥ 28 kg / m2), in association with a moderately hypocaloric, low-fat diet. The Committee recommended the granting of a marketing authorization for Alli.
Other information about Alli:
On 23 July 2007, the European Commission granted Glaxo Group Limited a "marketing authorization" for Orlistat GSK, valid throughout the European Union. This authorization was based on a previous authorization, granted in 1998, for Xenical ( capsules) The name of the medicine was changed to Alli on 12 September 2008.
Last update of this summary: 04-2009.
The information on Alli - orlistat published on this page may be out of date or incomplete. For a correct use of this information, see the Disclaimer and useful information page.




















-nelle-carni-di-maiale.jpg)




