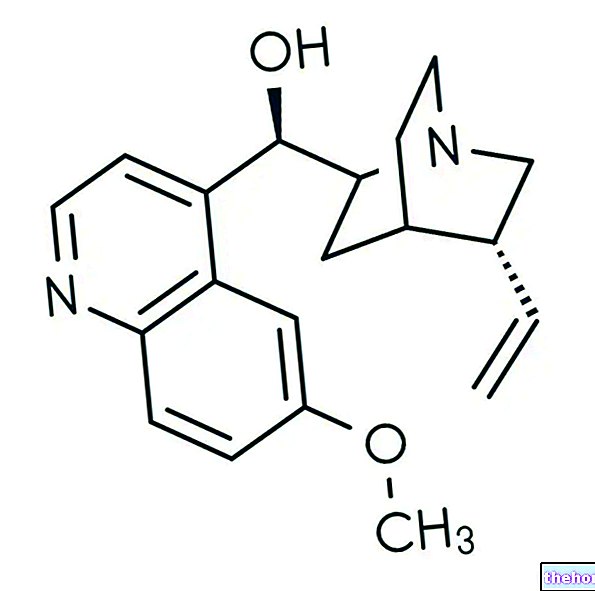
In detail, it is an inhibitor of the PARP enzyme (poly adenosine diphosphate-ribose polymerase or poly ADP-ribose polymerase). To carry out its action, olaparib must be taken orally, in fact, the medicines that contain it are formulated in the form of tablets or hard capsules.
In Italy, the active ingredient is available in a single medicinal product with the trade name Lynparza®. It is a medicine classified as a hospital drug that can be released in pharmacies (band H) which, in order to be dispensed, requires presentation of a non-repeatable limitative medical prescription (RNRL - drugs that can be sold to the public on prescription from hospitals or specialists).
BRCA-mutated, which responded to an initial standard platinum-based chemotherapy treatment.
On the other hand, olaparib tablets can be used for the treatment of:
- BRCA-mutated ovarian tumor that responded to first standard platinum-based chemotherapy treatment;
- Recurrent ovarian cancer, even when the cancer has responded to previous standard platinum-based chemotherapy treatment;
- Specific BRCA-mutated, HER2-negative, metastatic breast cancer (the cancer has spread to areas other than the developing area) after receiving chemotherapy treatments before or after the spread of the tumor.
Note: to understand if the tumor to be treated is BRCA-mutated, special tests are carried out.
(red blood cells, white blood cells, platelets) or if symptoms such as fever, infections, bruising or bleeding appear, as - although rarely - similar symptoms could indicate the presence of serious bone marrow problems (myelodysplastic syndrome - MDS, acute myeloid leukemia - LMA).