Food Sulfur is an essential component
Sulfur food it is an ORGANIC component naturally present both in food and in the human body (about 140g). This is a PLASTIC element, as it is part of the sulfur amino acids: methionine, cysteine and cystine; it is therefore also present in glutathione, coenzyme A, thiamine (vitamin B1), biotin (vit H) and insulin.

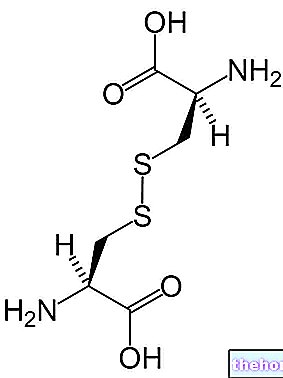
Chemical structure of cystine, a sulfur-containing amino acid
Furthermore, sulfur and the elements that contain it participate in the constitution of connective tissue, mucopolysaccharides and bile acids. An organic sulfur supplement, methylsulfonylmethane or MSM, is therefore proposed in the treatment of osteoarthritis.Dietary sulfur is absorbed in the small intestine while the main elimination routes are urine and faeces. Deficiency conditions are rare and the same is true for excess, which is likely to cause physical development disorders and insufficient growth.
Food sources of sulfur are mostly foods of protein origin (eggs, meat, fish and cheeses), while in the form of sulphates they can be introduced with drinking water and fruit and vegetables.
Sulphates: toxic compounds of the atmosphere and of contaminated food
Sulphates are INORGANIC compounds NOT NATURALLY present in food and their excessive concentration can be extremely harmful to human health.
Sulphates become toxic if they reach excessive concentrations; often, these are the result of the sum of the pollutants and of regularly used sulfates for technological processing; polluting sulphates can end up on food from atmospheric air or through polluted rains (acid rain), while the application of sulphates useful for food processing is subject to specific regulations for their use. Unfortunately, although regulated, the latter do not take into account the overall sulphates introduced, therefore their dietary intake should still be limited.
Overall, sulphates derive mainly from:
- Coal smoke (SO2 H2S)
- Combustion of petroleum (SO2, H2S)
- Sulfuric acid from industrial smog (H2SO4) and its lead salts (PbSO4)
- Food processing processes such as dehydration of fresh fruit (which uses sulfur to preserve color and some nutrients; manganese sulphate MnSO4)
- Foods, additives and drugs treated with sulfuric acid: saccharin, aspirin, alum (aluminum sulphate and potassium dodecahydrate KAl (SO4) 2 12 H2O, also known as potassium alum, is used in the industrial preparation of pickles and cherries in alcohol)
- Purification of water treated with copper sulphate (CuSO4)
- Use of insecticides based on tribasic copper sulphate
- Sulfonamide drugs (sulfonamides with a group characterized by a sulfur atom at valence 6; R-SO2-NH2)
- Saline purgatives: magnesium sulfate (MgSO4)
- Raising food additives (sulphates of: sodium, potassium, calcium, aluminum, aluminum and sodium, aluminum and potassium, aluminum and ammonium)
NB. It is important not to confuse sulphates (so far described) with food additives based on SULPHITES (SO32-) and SULFUR DIOXIDE; the latter are preservatives generally contained in wine, beverages, fish, dried fruit, etc.
More information on sulphites can be found in this article.
Toxicity of sulphates
Excessive amounts of sulphates in food can lead to a copper deficiency, otherwise sufficient to meet normal physiological needs.
Bibliography:
- Food chemistry - P. Cabras, A. Martelli - page 83
- Recommended Nutrient Intake Levels for the Italian Population (LARN ) - Italian Society of Human Nutrition (SINU)
- The complete book of minerals for health - J. I. Rodale - Dimetra - pag 140