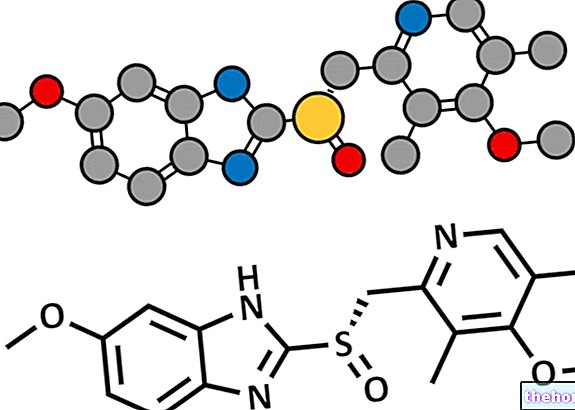
Paracetamol is a drug that is 95% deactivated through glucuronide and sulfur conjugation, and 5% through oxidation operated by Cyt P450, which gives rise to a highly reactive metabolite deactivated by glutathione. All this happens only if they are respected. therapeutic doses.
If the therapeutic doses are abundantly exceeded, the two types of conjugation are no longer sufficient to inactivate the paracetamol, so inactivation through Cyt P450 prevails. Consequently, there is a greater formation of reactive metabolites, which can cause necrosis of the liver cell.

- oxidation;
- hydrolysis;
- reduction of disulfide bridges (S - S);
- conjugation (sulfonic acid, glucuronic acid, acetic acid);
- introduction of functional groups (-OH or -COOH groups);
- bond with glutathione.
Detoxification cannot last long. In the previous case - that of paracetamol - with the exhaustion of the enzymes that inactivate the toxic, it remains in our body causing serious problems to the health of the liver cells. Furthermore, detoxification can slow down or even stop due to a possible decrease in substances. antioxidants (vitamins C and E), whose task is to block the development of oxygen free radicals.
We can conclude by saying that detoxification is a very useful process to preserve health, eliminating the toxic from our body; however, when some inactivation processes are lacking, the dangerous metabolite prevails, because it is not rendered inactive; consequently it is not eliminated from the organism.
Other articles on "Paracetamol and liver necrosis"
- Furosemide and liver damage
- Toxicity and toxicology
- Toxicodynamics