Generality
Cyanide is the chemical term that identifies any chemical compound containing the cyano group (CN).
The cyano group is a molecule formed by the union of a carbon atom and a nitrogen atom.

Examples of inorganic cyanide are hydrogen cyanide, sodium cyanide, potassium cyanide and cyanogen chloride.
Examples of organic cyanide, on the other hand, are nitriles, contained in the kernels of various fruits (apricots, peaches, cherries, etc.).
Cyanides are chemical substances that are widely used in manufacturing, from the paper industry to metallurgy, plastics, etc.
What is cyanide?
A cyanide is any chemical compound containing the cyano group (CN).
The cyano group is the result of the union of a carbon atom (the C of CN) to a nitrogen atom (the N of CN). The bond that unites carbon to nitrogen is a triple covalent bond. The total charge present on the CN group is negative, therefore, the cyano group is an anion.
CYANIDE AS POISON: INORGANIC CYANIDE
In the common imagination, the term cyanide refers to a powerful poison, with lethal effects.
Chemically, the cyanides that act as potent poisons are inorganic cyanides, such as sodium cyanide (NaCN), potassium cyanide (KCN) and cyanogen chloride (ClCN).
WHERE DOES INORGANIC CYANIDE COME FROM?
Any type of inorganic cyanide derives from the dissociation of hydrogen cyanide (HCN or hydrogen cyanide) or one of its salt (which can in turn be a cyanide).
Hydrogen cyanide is an inorganic molecule, the result of the union of a cyano group (CN) with a hydrogen atom (H). It is a highly toxic weak acid and can be considered a particular example of inorganic cyanide.
Please note: sodium cyanide and potassium cyanide are two salts of hydrogen cyanide.
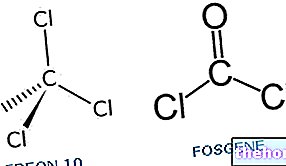
Cyanogen chloride, on the other hand, is a derivative of a salt of hydrogen cyanide; to be precise, it is a derivative of sodium cyanide. The oxidation of sodium cyanide with chlorine forms cyanogen chloride.
ORGANIC CYANIDE
In nature, the cyano anion CN also forms chemical compounds with organic molecules (eg: methyl groups, etc.), giving rise to types of organic cyanide.
Also known as nitriles, organic cyanides are mildly toxic or only become toxic on certain occasions; however, they are less and less poisonous than inorganic cyanides.

Property
The properties of a cyanide depend on which atoms are bonded to the CN cyano group.
Hydrocyanic acid is a pale blue or colorless liquid at room temperature, while it is a colorless gas at high temperatures. Both in liquid and gaseous form, it has an odor that resembles that of bitter almonds.
Sodium cyanide and potassium cyanide appear as white powders and give off - like hydrogen cyanide - an odor comparable to that of bitter almonds.
Cyanogen chloride is a colorless liquefied gas, heavier than air and with a particular pungent odor.
Where is it?
In nature, as well as in the kernels of some fruits, cyanide is also present in various plant species; plants keep it in the leaves and / or in the bark and use it to defend themselves from herbivores.
In addition to plants, other living things that produce cyanide (or substances containing the CN cyano group) are some bacteria and some fungi.
CYANIDE AS A COMBUSTION PRODUCT
Hydrogen cyanide is a possible product of combustion processes.
To be precise, it is present in the exhaust of internal combustion engines, in the cigarette smoke that is produced during the combustion of the latter and in the fumes deriving from the fusion of plastic materials based on acrylonitrile (an organic compound, containing a cyano group CN).
Uses
In manufacturing, cyanide is widely used. In fact, the paper industry (to produce paper), the textile industry (to produce fabrics, etc.), the industry for the production of plastic, the photographic industry (to produce all those chemical compounds for the development of the photos), the metallurgical industry (to produce steel and iron, to wash metals and for galvanization), the industry that deals with the treatment of waste water and the industry for the production of pesticides (against parasites, etc.) for various types of environment.
For obvious reasons, traces of cyanide may be found in the waste products of all the aforementioned activities.
PAST USES
In the past, hydrogen cyanide found use for tragic purposes.
For example, during the Second World War, Nazi Germany used it - under the name of Zyklon B - as a toxic agent in the gas chambers of the death camps.
Diffusion
Cyanide has the ability to spread in water, soil and air (N.B: in the air it is in the form of gas).
Humans can come into contact with cyanide by breathing contaminated air, drinking contaminated water, eating contaminated food or touching contaminated soil.
A "daily" source of cyanide - to which many people are exposed - is cigarette smoke.
Effects
After exposure, it takes little time for cyanide to enter the bloodstream and spread throughout the body via the blood.
The human body reacts to the presence of cyanide in different ways, depending on whether the doses are very low or medium-high.
When the doses are very low, cyanide transforms, through a series of cellular reactions, into thiocyanate, a chemical compound that is harmless to health and that the human being eliminates through the urine. Furthermore, always in low doses, cyanide combines with vitamin B12 and the resulting binomial seems to have beneficial effects on both nerve cells and blood cells.
When, on the other hand, the doses are medium to high, the ability of the human organism to transform cyanide into thiocyanate is suppressed (due to an excessive workload) and the toxic substance in question prevents the cells from using the oxygen. "oxygen leads to the death of the cells themselves.
The heart, the respiratory system and the central nervous system are most affected by a large exposure to cyanide.
Please note: it should be noted, for the avoidance of doubt, that chronic exposure to very small doses of cyanide can have toxic effects similar to those induced by exposure to medium-high doses.
TOXICITY
The toxic effects of cyanide on human health depend on three factors: the dose of toxic substance with which one comes into contact, the duration of exposure and the type of cyanide.
Typically, exposure to intermediate doses of cyanide involves:
- Acceleration of breathing;
- Sense of restlessness;
- Dizziness;
- Sense of weakness;
- Headache
- Nausea and feeling of vomiting;
- Acceleration of the heartbeat.
If the cyanide doses are high, the above manifestations are associated with:
- Convulsions;
- Hypotension;
- Slowing of the heart rhythm
- Loss of consciousness;
- Shortness of breath and difficulty breathing, due to lung problems
- Cardiac arrest.
The effects of exposure to cyanide begin to show themselves after just a few seconds / minutes. The dose mainly affects the speed of onset of the effects.
Typically, death from cyanide exposure occurs as a result of severe respiratory failure or cardiac arrest.
LONG-TERM EFFECTS
According to several clinical investigations, people who survive "massive cyanide exposure would tend to develop permanent neurological problems, some of which mimic symptoms very similar to those of Parkinson's disease."
LETHAL DOSE
When doses of cyanide are lethal, experts speak of "death from cyanide poisoning".
For sodium cyanide and potassium cyanide, the lethal dose for humans is 200-300 mg; for hydrogen cyanide, on the other hand, the lethal dose for humans is 50 mg.
Therapy
Exposure to toxic doses of cyanide represents a medical emergency to be treated with extreme speed and in the most appropriate manner.
In general, the very first therapeutic indications are:
- Move away as quickly as possible from the place / environment of exposure and move to an uncontaminated place;
- Take off your clothes, in the event that they are contaminated, and put them back in a plastic bag;
- Wash your eyes every 10-15 minutes if they burn;
- Wash any part of the skin that has come into contact with the cyanide with soap and water;
- Call for medical help.
When medical help arrives, they will complete the treatment, providing oxygen to the person concerned and an antidote.
The antidotes against exposure to large doses of cyanide are sodium thiosulfate, sodium nitrite and hydroxocobalamin.