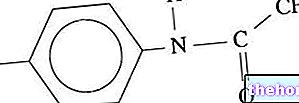
Once bioactivated, carbon tetrachloride (CCl4, a complex molecule and not very toxic in itself), leads to a chain of events and the formation of numerous toxic compounds. This molecule is also known by the name FREON 10.
Freon 10 was used in the past as a component in the liquid of fire extinguishers, refrigerators, air conditioners, stain removers and dry cleaning.
Freon 10 is very dangerous because it loses an electron when it is metabolized into trichloromethylene (CCl3); this last metabolite has an unpaired electron, so it becomes a radical, therefore a highly reactive compound. Trichloromethylene has many dangerous effects:
- it easily binds to the -EME group of proteins;
- blocks the activity of cytochrome P450 (since cytochrome is a "hemoprotein);
- acts on other cellular proteins;
- can cause liver necrosis and cancer;
- it can cause nephropathies;
- interacting with the fatty acids of the membrane lipids, it leads to the formation of chloroform, which if oxidized leads to the formation of phosgene.
In the past, chloroform was used as an anesthetic; it is actually a very toxic substance because it leads to the formation of a very toxic carbon tetrachloride metabolite.

Also from Freon 10 we obtain PHOSGENE, which is a highly toxic metabolite. In particular, of all the metabolites that are formed with the metabolization of Freon 10, Phosgene is clearly the most toxic compared to the others.
The Phosgene metabolite in our body is inhibited with a "hydrolysis action that splits it into hydrochloric acid (HCl) and carbon dioxide (CO2). However, if this inhibition system is insufficient, the Phosgene binds irreversibly through covalent bonds. to all proteins.
Other articles on "Carbon tetrachloride or Freon 10"
- Dibromoethane: health effects
- Toxicity and toxicology
- Aromatic amines and food cooking