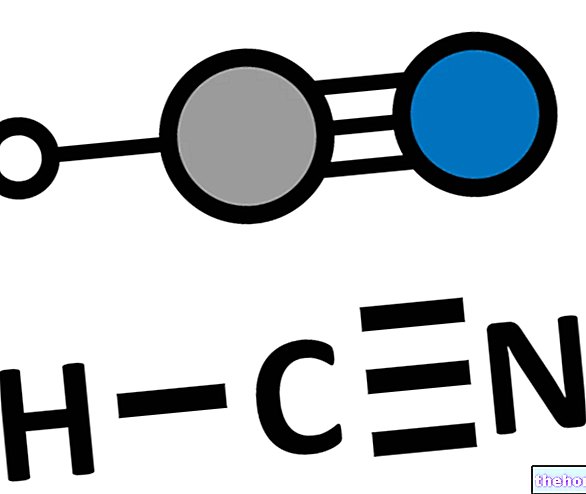
To have a toxic activity, benzene must undergo a bioactivation process. The metabolites responsible for the toxic and carcinogenic action are free radicals.
Free radicals (especially oxygen free radicals) are energetically unstable and highly reactive molecules. Benzene undergoes an oxidation reaction by the hepatic microsomal system, therefore by cytochrome P450, with the introduction of two hydroxyl groups - OH. The hydroquinone originated from the oxidation of benzene becomes a very important substrate of an enzyme present in the bone marrow. The enzyme is a myeloperoxidase which catalyzes the transformation of this hydroquinone into a radical.

The free radical that is formed derives from a break in the bond between oxygen and hydrogen of the hydroxyl group. Oxygen has an unpaired electron released by hydrogen during the breaking of the bond, thus giving oxygen strong reactive characteristics. The activity that this radical has is very dangerous because it blocks the production of cells in the bone marrow.
Other articles on "Benzene: Health Effects"
- Parathion: health effects
- Toxicity and toxicology
- Polycyclic aromatic hydrocarbons