
What is Valdoxan?
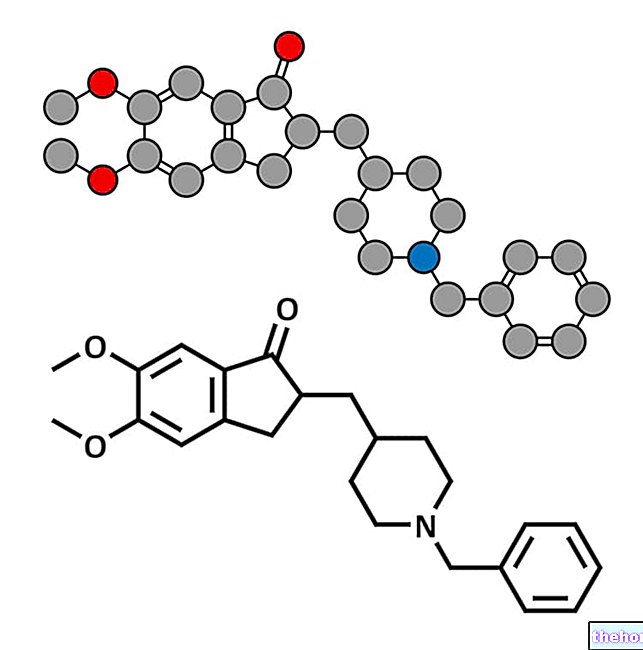
Valdoxan is a medicine that contains the active substance agomelatine and is available as yellow-orange elongated tablets (25 mg).
What is Valdoxan used for?
Valdoxan is used to treat major depression in adults. Major depression is a disease that causes the sufferer to have mood disorders that interfere with daily life. Symptoms often include deep sadness, a sense of worthlessness, loss of interest in favorite activities, sleep disturbances, a sense of slowing down , feelings of anxiety and weight changes.
The medicine can only be obtained with a prescription.
How is Valdoxan used?
The recommended dose of Valdoxan is one tablet a day, taken at bedtime, with or without food. If after two weeks there is no improvement in symptoms, your doctor may increase the dose to two tablets, taken together before going to bed. Depressed patients should be treated for at least six months to ensure the disappearance of symptoms.
The patient's liver should be monitored by carrying out a blood test at the start of treatment and subsequently after approximately 6, 12 and 24 weeks. Liver checks should also be performed in case of symptoms that could indicate the onset of liver problems. Treatment should be stopped if blood tests reveal abnormal levels of liver enzymes. In this case, the blood test will have to be repeated until these levels have returned to normal.
As a positive effect of Valdoxan in patients over 65 has not been clearly demonstrated, the medicine should be used with caution in patients of this age group. It should also be used with caution in patients with severe or moderate kidney problems. The medicine should not be used in patients with liver problems.
How does Valdoxan work?
The active ingredient in Valdoxan, agomelatine, is an antidepressant and works in two ways: by stimulating the MT1 and MT2 receptors, usually activated by melatonin, and by blocking the 5-HT2C receptors, usually activated by the neurotransmitter 5-hydroxytryptamine (known also as "serotonin"). This is believed to lead to increased levels of dopamine and norepinephrine among nerve cells in areas of the brain involved in mood control. This is believed to help relieve symptoms of depression. Valdoxan could also be used to normalize the patient's sleep stages.
How has Valdoxan been studied?
The effects of Valdoxan were first tested in experimental models before being studied in humans.
Valdoxan has been compared with placebo (a dummy treatment) in five main short-term studies involving a total of 1,893 adults with major depression. Three of the studies included patients treated with other antidepressants, fluoxetine or paroxetine, as an "active comparator". The active comparator groups were included to check the study's ability to measure the effectiveness of drugs in treating depression. The main measure of effectiveness of all five studies was the change in symptoms after six weeks, as measured by a standard scale for depression, the Hamilton Depression Rating Scale (or HAM-D, Hamilton's scale for evaluating depression). The company also presented the results of an additional study that compared Valdoxan with sertraline (another antidepressant).
Two other main studies compared the ability of Valdoxan and placebo to prevent symptoms from returning in 706 patients with depression previously controlled with Valdoxan. The main measure of effectiveness was the number of patients with symptoms returning over 24-26 weeks of treatment.
What benefit has Valdoxan shown during the studies?
In the short-term studies, Valdoxan was more effective than placebo in the two studies where no active comparator was used. In the other three studies, in which an active comparator was used, there was no difference in scores between patients treated with Valdoxan and those treated with placebo. However, in two of these studies no effects of fluoxetine or paroxetine were observed, making the results difficult to interpret. The further study indicated that agomelatine was more effective than sertraline, with a difference in HAM-D score of 1.68 after six weeks.
In the first of the long-term studies, there was no difference between Valdoxan and placebo in preventing symptoms from returning during the 26 weeks of treatment. However, the second study showed that symptoms returned in 21% of patients treated with Valdoxan over the course of 24 weeks (34 out of 165), compared with 41% of patients treated with placebo (72 out of 174).
What is the risk associated with Valdoxan?
The most common side effects of Valdoxan (seen in 1 to 10 patients in 100) are headache, dizziness, sleepiness, insomnia, migraine, nausea, diarrhea, constipation, pain in the upper abdomen (stomach pain), hyperhidrosis (excessive sweating), back pain, fatigue, increased liver enzymes and anxiety. Most side effects were mild or moderate and occurred within the first two weeks of treatment. Some of these may be linked to the patient's depression. than to Valdoxan itself. For the full list of side effects reported with Valdoxan, see the package leaflet.
Valdoxan should not be used in patients who may be hypersensitive (allergic) to agomelatine or any of the other ingredients. Valdoxan should not be used in patients with liver problems, such as cirrhosis (scarring of the liver) or active liver disease. It must also not be used in patients taking medicines that slow down the breakdown of Valdoxan in the body, such as fluvoxamine (another antidepressant) and ciprofloxacin (an antibiotic).
Valdoxan should also not be used in elderly patients with dementia.
Why has Valdoxan been approved?
The Committee for Medicinal Products for Human Use (CHMP) noted that the benefits of Valdoxan in the treatment of depression may be less than those seen with other antidepressants. However, considering that the medicine has a new mode of action, a limited number of side effects and a different safety profile than existing antidepressants, the Committee concluded that Valdoxan could be a valuable treatment for some patients. provided that liver function is monitored frequently. The CHMP therefore decided that Valdoxan's benefits outweigh its risks in the treatment of major depressive episodes in adults. The Committee recommended that Valdoxan be given a marketing authorization.
What measures are being taken to ensure the safe use of Valdoxan?
At the time of commercialization of Valdoxan, the company that produces it will provide training materials for those who prescribe it. These materials will illustrate the safety of the medicine, in particular the possible effects on the liver and interactions with other medicines.
Other information about Valdoxan:
On 19 February 2009, the European Commission granted Les Laboratoires Servier a "Marketing Authorization" for Valdoxan, valid throughout the European Union.
For the full version of Valdoxan's EPAR, click here.
Last update of this summary: 12-2008.
The information on Valdoxan - agomelatine published on this page may be out of date or incomplete. For a correct use of this information, see the Disclaimer and useful information page.



















-nelle-carni-di-maiale.jpg)




