
What is Dasselta - Desloratadine?
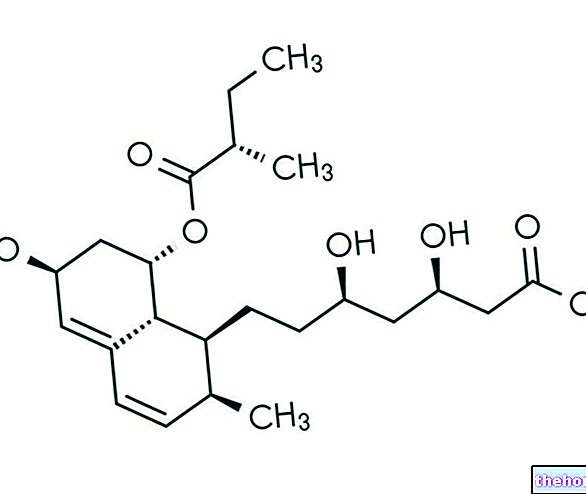
Dasselta is a drug containing the active ingredient desloratadine. The medicine is available in the form of tablets (5 mg).
Dasselta is a 'generic medicine'. This means that Dasselta is similar to a 'reference medicine' already authorized in the European Union (EU) called Aerius.
What is Dasselta - Desloratadine used for?
Dasselta is used for the relief of symptoms of allergic rhinitis (inflammation of the nasal passages caused by an allergy, such as hay fever or dust mite allergy) or hives (skin condition caused by an allergy) , symptoms of which include itching and rash).
The medicine can only be obtained with a prescription.
How is Dasselta used - Desloratadine?
The recommended dose for adults and adolescents (12 years and older) is one tablet once a day.
How does Dasselta - Desloratadine work?
Desloratadine, the active ingredient in Dasselta, is an antihistamine. It works by blocking the receptors on which histamine, a substance present in the body that causes allergic symptoms, is normally fixed. Once the receptors are blocked, histamine fails to produce its effect and therefore a decrease in allergy symptoms is observed.
How has Dasselta - Desloratadine been studied?
Because Dasselta is a generic medicine, the patient studies have been limited to tests to determine that the medicine is bioequivalent to the reference medicine, Aerius. Two medicines are bioequivalent when they produce the same levels of the active substance in the body.
What are the benefits and risks of Dasselta - Desloratadine?
Because Dasselta is a generic medicine and is bioequivalent to the reference medicine, its benefits are taken as being the same as the reference medicine's.
Why has Dasselta - Desloratadine been approved?
The CHMP concluded that, in accordance with EU requirements, Dasselta has been shown to have comparable quality and to be bioequivalent to Aerius. Therefore, the CHMP considered that, as in the case of Aerius, the benefits outweigh the identified risks and recommended the issuing of the marketing authorization for Dasselta.
More information about Dasselta - Desloratadine
On November 28, 2011, the European Commission issued a "Marketing Authorization" for Dasselta, valid throughout the European Union.
For more information about Dasselta therapy, read the package leaflet (included with the EPAR) or contact your doctor or pharmacist.
Last update of this summary: 10-2011.
The information on Dasselta - Desloratadine published on this page may be out of date or incomplete. For a correct use of this information, see the Disclaimer and useful information page.




















-nelle-carni-di-maiale.jpg)




