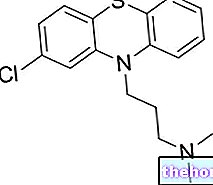
Active ingredients: Paroxetine
STILIDEN 10 mg / ml oral drops solution
Stiliden package inserts are available for pack sizes:- STILIDEN 10 mg / ml oral drops solution
- Stiliden 20 mg film-coated tablets
Why is Stiliden used? What is it for?
STILIDEN is a therapy for adults with depression and / or anxiety disorders such as: obsessive compulsive disorders, panic disorders (panic attacks), social anxiety disorder (fear or flight from social situations), post-traumatic stress disorder, generalized anxiety disorder.
STILIDEN is part of the group of drugs called SSRIs (selective serotonin reuptake inhibitors).
Every person has a substance called serotonin in their brain. People who are depressed or anxious have lower levels of serotonin than others. It is not entirely clear how STILIDEN and the other SSRIs work, but they may be helpful by increasing the level of serotonin in the brain.
Other medications or psychotherapy can also treat depression and anxiety. Proper treatment of depression and anxiety disorders is important to help you get better. If left untreated, your disease may not heal and can become more severe and more difficult to treat.
You may find it helpful to tell a friend or relative about your depression or anxiety disorder and ask them to read this leaflet. You may ask them to tell you if they think your depression or anxiety is. getting worse or are worried about changes in your behavior.
Contraindications When Stiliden should not be used
Do not take STILIDEN
- If you have ever had an allergic reaction to paroxetine or to any of the ingredients listed. See section 6 "Package contents and other information".
- If you are taking medicines called monoamine oxidase inhibitors (MAOIs including moclobenide), or have taken them in the past two weeks. Your doctor will advise you on how you should start taking STILIDEN after you have stopped your MAOI.
- If you are taking a tranquilizer called thioridazine.
- If you are taking an antipsychotic called pimozide.
If any of these apply to you, talk to your doctor without taking STILIDEN.
Precautions for use What you need to know before taking Stiliden
Check with your doctor
- If you are taking other medicines (see "Other medicines and STILIDEN").
- If you have eye, kidney, liver or heart problems.
- If you have epilepsy or have had seizures.
- If you have episodes of mania (manic behaviors or thoughts).
- If you are having electroconvulsive therapy (ECT).
- If you have had a bleeding disorder.
- If you are taking tamoxifen to treat breast cancer or fertility problems, STILIDEN can make tamoxifen less effective, therefore your doctor may recommend that you take another antidepressant.
- If you have diabetes.
- If you are on a low sodium diet.
- If you have glaucoma (increased pressure in the eye).
- If you are pregnant or planning to become pregnant (see Pregnancy, breast-feeding and fertility inside this leaflet).
In these cases, and if you have not already discussed it with your doctor, go back to your doctor and ask what to do about taking STILIDEN.
Thoughts of suicide and worsening of depressive or anxiety disorder
If you are depressed and / or have anxiety disorders, you may sometimes have thoughts of harming or killing yourself. These may increase if you are taking antidepressants for the first time, as these drugs take some time to work, usually about two weeks but sometimes even more.
He may have a greater predisposition towards these thoughts:
- If you have previously had thoughts of killing or harming yourself
- If you are a young adult. Clinical studies have shown an increased risk of suicidal behavior in adults under the age of 25 with psychiatric conditions treated with an antidepressant.
If at any time you have thoughts of killing or harming yourself, contact your doctor or go to a hospital immediately. You may find it helpful to tell a friend or relative about your depression or anxiety disorder and ask them to read this leaflet. You may ask them to tell you if they think your depression or anxiety is. getting worse or are worried about changes in your behavior.
Interactions Which drugs or foods can modify the effect of Stiliden
Some medicines can affect the way STILIDEN works or make it easier for you to have side effects. STILIDEN can also affect the way some other medicines work.
These include:
- Medicines called monoamine oxidase inhibitors (MAOIs, including moclobemide). See "Do not take STILIDEN" in this leaflet.
- Thioridazine or pimozide, antipsychotic drugs. See "Do not take STILIDEN" in this leaflet.
- Aspirin, ibuprofen and other drugs called NSAIDs (Non-Steroidal Anti-Inflammatory Drugs) such as celecoxib, etodolac, meloxicam and refecoxib, used for pain and inflammation.
- Pain relievers tramadol and pethidine.
- Medicines called triptans such as sumatriptan used to treat migraines.
- Other antidepressants including other SSRIs, tryptophan and tricyclic antidepressants such as clomipramine, nortriptyline and desipramine.
- Medicines such as lithium, risperidone, perphenazine, pimozide (called antipsychotics or neuroleptics) used to treat some psychiatric disorders.
- St. John's wort is a herbal remedy for depression.
- Atomoxetine used to treat attention deficit hyperactivity disorder (ADHD).
- Phenobarbital, phenytoin or carbamazepine used to treat seizures or epilepsy.
- Procyclidine, used to relieve tremor especially in Parkinson's disease.
- Warfarin or other medicines (called anticoagulants) used to thin the blood.
- Propafenone, flecainide and drugs used for arrhythmias (irregular heartbeat).
- Metoprolol, a beta blocker used to treat high blood pressure and heart ailments.
- Pravastatin, used to treat high cholesterol levels.
- Rifampicin used to treat tuberculosis (TB) and leprosy.
- Linezolide an antibiotic.
- Fentanyl, used in anesthesia or to treat chronic pain.
- A combination of fosamprenavir and ritonavir, used to treat Human Immunodeficiency Virus (HIV) infection.
- Tamoxifen, used to treat breast cancer or fertility problems. If you are taking a medicine from this list and have not yet discussed it with your doctor, go back to your doctor and ask what to do. You may need to change your dosage or change your medicine.
If you are taking a medicine from this list and have not yet discussed it with your doctor, go back to your doctor and ask what to do. You may need to change your dosage or change your medicine.
If you are taking any other medicines, even those without a prescription, ask your doctor or pharmacist before taking STILIDEN. They will be able to tell you if it is safe to take STILIDEN in these cases.
Taking STILIDEN with alcohol
Do not drink alcohol while you are taking STILIDEN. Alcohol can make your symptoms or side effects worse.
Warnings It is important to know that:
Pregnancy, breastfeeding and fertility
Talk to your doctor immediately if you are pregnant, if you may be inciting or planning to become pregnant. In infants whose mothers had taken STILIDEN during the first months of pregnancy, there was evidence of an increased risk of birth defects, particularly heart defects. In the general population, about 1 in 100 newborns is born with a heart defect.
This event increases to 2 in 100 infants in mothers who have taken STILIDEN.
You and your doctor will decide whether it is better for you to discontinue STILIDEN gradually during pregnancy. However, based on your clinical picture, your doctor may suggest that it is best to continue taking Stiliden.
Make sure your midwife or doctor knows you are taking STILIDEN. When medicines such as STILIDEN are taken during pregnancy, particularly during the last three months of pregnancy, they may increase the baby's risk of a serious condition, called persistent pulmonary hypertension in the newborn (PPHN). In PPHN, the blood pressure in the blood vessels between the baby's heart and the lungs is too high. If you take STILIDEN during the last three months of pregnancy, your baby may also have other symptoms which usually occur during the first 24 hours after birth.
These symptoms include:
- Respiratory problems
- Skin that is bluish or too hot or too cold
- Blue lips
- Vomiting or difficulty in feeding
- Tiredness, inability to sleep, or widespread crying
- Stiff or flabby muscles
- Tremors, nervousness or convulsions
If your baby has any of these symptoms at birth or if you are concerned about your baby's health, contact your doctor or midwife who will be able to assist you.
STILIDEN can pass in very small quantities into breast milk. If you are taking STILIDEN, go back to your doctor and talk to him before you start breastfeeding. You and your doctor may decide that you can breastfeed while you are taking STILIDEN.
Effect on male fertility
Medicines such as STILIDEN can reduce the quality of sperm. Although the impact on fertility is not known, in some men while taking STILIDEN, fertility may be impaired.
Driving and using machines
STILIDEN can cause dizziness, confusion and visual disturbances. If you experience these side effects, do not drive or use machines.
Important information about some of the ingredients of STILIDEN
This product contains sucrose, so if your doctor has advised you that you have an intolerance to some sugars, contact your doctor before taking STILIDEN.
The product contains 3.3% v / v of ethanol (contained in the anise flavor). Therefore a dose of 1 ml of STILIDEN contains the equivalent of less than 1 ml of beer and 0.3 ml of wine (6 ml are equivalent to 4 ml of beer and 1.6 ml of wine). Therefore, people suffering from alcoholism, pregnant or breastfeeding women, children and patients with liver disease should be careful.
This product does not contain gluten and can be taken by people with celiac disease.
Sportsmen: the medicine contains ethanol therefore it can give positive anti-doping tests.
Dose, Method and Time of Administration How to use Stiliden: Posology
Take STILIDEN drops, diluted in water, in the morning with breakfast.
It is important to take your medicine as prescribed by your doctor who will advise you which dose to take when you first start STILIDEN. Most people start feeling better after a couple of weeks. If you do not start to feel better after this period, please inform your doctor, who may decide to gradually increase the dose, up to the maximum allowed daily dose.
The usual doses for the different indications are shown in the table below.
Your doctor will inform you about the daily dose and how long you will need to take the medicine for. It could be for several months or more.
Use in children and adolescents
STILIDEN should not be used by children and adolescents under the age of 18 because it has not been shown to be effective for these age groups. In addition, patients under the age of 18 have an increased risk of side effects such as suicidal thoughts and self-harm when taking STILIDEN. If your doctor has prescribed STILIDEN for you (or your child) and you want to discuss it, please go back to your doctor.
In studies with STILIDEN less than 1 in 10 patients under the age of 18 developed an increase in suicidal thoughts and suicide attempts, self-harm, hostility, aggression or grumpiness, loss of appetite, shaking, abnormal sweating, hyperactivity (excessive energy ), agitation, emotional changes (including crying and mood changes) and unexpected bruising or bleeding (eg nosebleeds). These studies have also shown that the same symptoms appear in children and adolescents who take sugar-containing tablets (placebo ) instead of STILIDEN, albeit less frequently.
In these studies, some patients under the age of 18 experienced withdrawal effects similar to those seen in adults after stopping treatment with STILIDEN. In addition, less than 1 in 10 patients under the age of 18 experienced stomach pain. nervousness and emotional changes (including crying, mood swings, self-harm, suicidal thoughts and suicide attempts).
Elderly patients
The maximum allowable dose for people over 65 years of age is 4 ml per day.
Patients with liver or kidney disorders
If you have severe liver or kidney problems, your doctor may decide that you may need a lower dose than normal.
If you forget to take STILIDEN
Take your medicine at the same time each day. If you forget to take a dose and remember before going to sleep, take it immediately and continue your therapy as normal the next day. If you only remember during the night or the next day, do not take the missed dose. You may experience withdrawal effects, but these disappear after you take your usual dose at the usual time.
If you stop using STILIDEN
Do not stop taking STILIDEN until your doctor tells you to.
When you stop treatment, your doctor will help you reduce the dose slowly over a few weeks or months to reduce the risk of withdrawal effects. One way to do this is to gradually reduce the dose of STILIDEN you take by 10 mg per week. Most people find withdrawal symptoms to be mild and disappear spontaneously within two weeks. For some people, these symptoms may be more severe or last longer. If you experience withdrawal effects when you stop taking the drops, your doctor may decide to stop taking the medicine more slowly. If you experience severe withdrawal effects when you stop taking STILIDEN, please contact your doctor. Your doctor may ask you to start taking the drops again and stop treatment more slowly.
Despite the withdrawal effects, you will still be able to discontinue STILIDEN.
Possible withdrawal effects when stopping treatment
Studies show that 3 out of 10 patients report one or more symptoms upon stopping treatment with STILIDEN. Some withdrawal symptoms occur more frequently than others.
Symptoms that may affect up to 1 in 10 people:
- Feeling dizzy, unsteady or unbalanced.
- Tingling sensation, burning sensation and (less commonly) electric shock sensation, including in the head.
- Some patients have experienced ringing, hissing, whistling, ringing or other persistent noises in the ear (tinnitus).
- Sleep disturbances (restless dreams, nightmares, difficulty falling asleep).
- Anxiety.
- Headache.
Symptoms that may affect up to 1 in 100 people:
- Feeling sick (nausea).
- Sweating (including night sweating).
- Restlessness or agitation.
- Tremors (shaking).
- Confusion or disorientation.
- Diarrhea (loose stools).
- Increased emotionality or irritability.
- Visual disturbances.
- Changes in heartbeat (palpitations).
Tell your doctor if you are concerned about these withdrawal effects when you stop treatment with STILIDEN.
What should he do if he is not improving
STILIDEN will not improve your symptoms immediately, all antidepressants need time to work. Some people start to feel better within a couple of weeks, others need more time. If you have not started to improve after a couple of weeks, go to your doctor who will tell you what to do. Some people who take antidepressants feel worse before they get better. Your doctor should see you again a couple of weeks after you have started treatment. Tell your doctor if it hasn't started to improve.
Overdose What to do if you have taken too much Stiliden
If you or someone else takes too many drops of STILIDEN, in addition to the symptoms listed in Section 4 "POSSIBLE SIDE EFFECTS" you may have vomiting, dilated pupils, fever, changes in blood pressure, headache, involuntary muscle twitching, agitation, anxiety and heart rate faster than normal.
In any case, tell your doctor or go to hospital immediately, taking the medicine bottle with you.
Side Effects What are the side effects of Stiliden
Like all medicines, STILIDEN can cause side effects, although not everybody gets them.
If you have any of the following side effects during treatment, contact your doctor or go to a hospital immediately.
Uncommon (present in 1 in 100 patients)
- If you have bruising without a specific cause or bleeding, including blood in your vomit or stool.
- If you find it difficult to urinate.
Rare (present in 1 in 1000 patients)
- If you have convulsions.
- If you are restless or unable to sit or stand still, you may have what is called akathisia. Increasing your dose of STILIDEN could make these symptoms worse.
- If you feel tired, weak or confused and have muscle pain, stiffness or incoordination, this could be due to a rare effect of STILIDEN which can lead to a lack of sodium in your blood.
Very rare (affects 1 in 10,000 patients)
- Allergic reactions to STILIDEN, which can be serious, If you develop an allergic reaction, redness and rash, swelling of the eyelids, face, lips, mouth or tongue, itching or difficulty in breathing (shortness of breath) or swallowing and if you feel faint or dizzy resulting in collapse or loss of consciousness, contact your doctor or go to the nearest hospital immediately.
- If you have serotonin syndrome or neuroleptic malignant syndrome. Symptoms include: confusion, restlessness, sweating, shaking, shaking, hallucinations (strange sights or sounds), sudden muscle twitching, or a fast heartbeat.
- If you develop acute glaucoma (your eyes become painful and you have blurred vision).
Other possible less serious side effects that may appear during treatment
Very common (present in more than 1 in 10 patients)
- Feeling sick (nausea). Taking this medicine in the morning with breakfast will reduce the chance of these symptoms.
- Changes in sexual habits or sexual functions. For example, lack of orgasm and, in men, abnormal erection and ejaculation.
Common (present in 1 out of 10 patients)
- Increase in the level of cholesterol in the blood
- Loss of appetite.
- Disturbed sleep (insomnia) or sleepiness.
- Abnormal dreams (including nightmares).
- Dizziness or shaking (tremors).
- Difficulty concentrating.
- Headache.
- Feeling agitated.
- Blurred vision.
- Yawns, dry mouth.
- Diarrhea or constipation.
- He retched.
- Weight gain.
- Feeling weak.
- Sweating.
Uncommon (present in 1 in 100 patients)
- Transient increases or decreases in blood pressure, heart rate faster than normal.
- Inability to move, stiffness, shaking or abnormal movements of the mouth and tongue.
- Dilation of the pupils.
- Skin rashes.
- Confusion.
- Hallucinations (strange visions and sounds).
- Drop in blood pressure following the transition from a lying or sitting position to a standing position, with dizziness, fainting and possible visual disturbances.
- Inability to urinate (water retention) or uncontrolled and involuntary loss of urine (urinary incontinence).
- If you are a diabetic patient, you may notice an alteration in your blood glucose levels while being given STILIDEN. In these cases, please contact your doctor who will explain how to adjust the dosage of your insulin or other medicines you use to treat diabetes.
Rare (present in 1 in 1000 patients)
- Abnormal milk production in the mammary glands of men and women.
- Slow heartbeat.
- Liver changes shown in liver specific blood tests.
- Panic attacks.
- Manic behavior or thoughts.
- Feeling of detachment from one's body (depersonalization).
- Anxiety.
- Irresistible urge to move the legs (Restless Legs Syndrome).
- Joint or muscle pain.
Very rare (affects 1 in 10,000 patients
- Liver problems that make your skin or the whites of your eyes yellow.
- Water and fluid retention which can cause swelling of the arms or legs.
- Sensitivity to sunlight.
- Severe skin reactions.
- Continuous and painful erection of the penis.
- Unexpected bleeding e.g. bleeding from the gums, blood in the urine or vomit, or unexpected bruising or rupture of blood vessels (ruptured veins).
- Some patients have complained of ringing, hissing, whistling, ringing or other persistent noises in the ear (tinnitus) when taking STILIDEN.
- An increased risk of bone fractures has been observed in patients taking this type of medication.
Frequency not known (frequency cannot be estimated from the available data)
- aggression
If you have any concerns while taking STILIDEN, talk to your doctor and / or pharmacist who will be able to advise you.
Reporting of side effects
If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system at: www.agenziafarmaco.gov.it/it/responsabili. By reporting side effects you can help provide more information on the safety of this medicine.
Expiry and Retention
- Keep STILIDEN out of the reach and sight of children.
- Do not use STILIDEN after the expiry date which is stated on the carton after the expiry date. The expiry date refers to the last day of the month.
- After first opening the bottle, the oral solution is valid for 30 days for the 30 ml bottle and 60 days for the 60 ml bottle.
- Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. This will help protect the environment.
Deadline "> Other information
Composition
The active ingredient is paroxetine as hydrochloride.
The other ingredients are: hydroxypropylbetadex, sucrose, anise flavor (anethole, water, ethanol), sodium benzoate E211, purified water, 1N hydrochloric acid.
Description of the appearance of STILIDEN and contents of the pack
Each carton contains a 30 ml or 60 ml bottle and a graduated pipette.
Each ml of liquid (20 drops) contains 10 mg of paroxetine.
Source Package Leaflet: AIFA (Italian Medicines Agency). Content published in January 2016. The information present may not be up-to-date.
To have access to the most up-to-date version, it is advisable to access the AIFA (Italian Medicines Agency) website. Disclaimer and useful information.
01.0 NAME OF THE MEDICINAL PRODUCT -
STILIDEN 10 MG / ML ORAL DROPS, SOLUTION
02.0 QUALITATIVE AND QUANTITATIVE COMPOSITION -
Each ml (1 ml corresponds to 20 drops) of STILIDEN contains:
paroxetine HCl 11.11 mg (corresponding to 10 mg of paroxetine base).
For the full list of excipients see section 6.1.
03.0 PHARMACEUTICAL FORM -
Oral drops.
Bottle of 30 and 60 ml.
04.0 CLINICAL INFORMATION -
04.1 Therapeutic indications -
Treatment of
• Major depressive episode
• Obsessive Compulsive Disorder
• Panic disorder with or without agoraphobia
• Social anxiety disorder / social phobia
• Generalized anxiety disorder
• Post-traumatic stress disorder
04.2 Posology and method of administration -
The bottle is supplied with a 1 ml graduated dropper (1 ml corresponds to 20 drops equal to 10 mg of paroxetine free base).
1 drop corresponds to 0.5 mg of paroxetine free base.
It is recommended to administer STILIDEN drops in a single intake in the morning during breakfast. The drops should be diluted in water.
EPISODES OF MAJOR DEPRESSION
The recommended dose is 20 mg once a day. In general, improvement in patients begins after one week but may only become evident from the second week of therapy.
As with all antidepressant drugs, the dosage should be reviewed and adjusted as necessary within the first three to four weeks after initiation of therapy and thereafter as deemed clinically appropriate.
In some patients, who have an insufficient response to the 20 mg dose, the dose may be gradually increased up to a maximum of 50 mg per day, in 10 mg increments, based on the patient's response.
Patients with depression should be treated for a sufficient period of at least six months to ensure they are symptom-free.
OBSESSIVE COMPULSIVE DISORDER
The recommended dose is 40 mg per day. Patients should be started on a dose of 20 mg per day and the dose can be gradually increased in 10 mg increments up to the recommended dose. If after a few weeks there is insufficient response to the recommended dose, some patients may benefit from gradually increasing the dose up to a maximum of 60 mg per day.
Patients with OCD should be treated for a sufficient period to ensure they are symptom-free. This period can be several months or even longer (see section 5.1 Pharmacodynamic properties).
PANIC DISORDER
The recommended dose is 40 mg per day. Patients should start on a dose of 10 mg per day and the dose gradually increased, with 10 mg increases to the recommended dose, based on the patient's response.
A low starting dose is recommended to minimize the potential for worsening of panic symptoms, as has generally been observed in the initial treatment of this disorder.
If after a few weeks there is insufficient response to the recommended dose, some patients may benefit from gradually increasing the dose up to a maximum of 60 mg per day.
Patients with panic disorder should be treated for a sufficient period to ensure they are symptom-free. This period can be several months or even longer (see section 5.1 Pharmacodynamic properties).
SOCIAL ANXIETY / SOCIAL PHOBIA DISORDER
The recommended dose is 20 mg per day. If insufficient response to the recommended dose is observed after a few weeks, some patients may benefit from gradually increasing their dose in 10 mg increments up to a maximum of 50 mg per day. Long-term use should be considered. periodically (see section 5.1 Pharmacodynamic properties).
GENERALIZED ANXIETY DISORDER
The recommended dose is 20 mg per day. If after a few weeks there is insufficient response to the recommended dose, some patients may benefit from gradually increasing their dose in 10 mg increments up to a maximum of 50 mg per day.
Long-term use should be evaluated periodically (see section 5.1 Pharmacodynamic properties).
POST-TRAUMATIC STRESS DISORDER
The recommended dose is 20 mg per day. If after a few weeks there is insufficient response to the recommended dose, some patients may benefit from gradually increasing their dose in 10 mg increments up to a maximum of 50 mg per day.
Long-term use should be evaluated periodically (see section 5.1 Pharmacodynamic properties).
GENERAL INFORMATIONS
WITHDRAWAL SYMPTOMS OBSERVED ON DISCONTINUATION OF THE
TREATMENT WITH PAROXETINE
Abrupt discontinuation of treatment should be avoided (see section 4.4 Special warnings and special precautions for use and section 4.8 Undesirable effects).
The tapering regimen used in clinical trials used a tapering daily dose of 10 mg at weekly intervals.
If intolerable symptoms occur following dose reduction or upon discontinuation of treatment, resuming the previously prescribed dose may be considered. Thereafter, the doctor may continue to reduce the dose but more gradually.
Special populations:
• Senior citizens
Increased plasma concentrations of paroxetine have been observed in elderly subjects, however the range of plasma concentrations is similar to that seen in younger subjects.
Treatment should start at the same doses as in adults. In some patients, increasing the dose may be useful, but the maximum dose should not exceed 40 mg per day.
• Children and adolescents (7-17 years)
Paroxetine should not be used for the treatment of children and adolescents as it has been found in controlled clinical trials that paroxetine is associated with an increased risk of suicidal behavior and hostile behavior. Furthermore, efficacy was not adequately demonstrated in these studies (see section 4.4 Special warnings and special precautions for use and section 4.8 Undesirable effects).
• Children under the age of 7
The use of paroxetine in children less than 7 years of age has not been studied. Paroxetine should not be used until safety and efficacy in this age group have been established.
• Renal / hepatic insufficiency
Increased plasma concentrations of paroxetine have been reported in patients with severe renal insufficiency (creatinine clearance less than 30 ml / min) or in patients with hepatic insufficiency. Therefore, the dosage should be limited to the lowest doses of the dosage range.
04.3 Contraindications -
Known hypersensitivity to paroxetine or to any of the excipients.
Paroxetine is contraindicated in combination with monoamine oxidase inhibitors (MAO inhibitors).
In exceptional cases, linezolid (an antibiotic that is a reversible non-selective MAO-inhibitor) can be administered in combination with paroxetine provided that careful observation of the symptoms of serotonin syndrome and monitoring of blood pressure in facilities with adequate equipment is possible. (see section 4.5)
Paroxetine treatment can be initiated:
- two weeks after stopping treatment with a non-reversible MAO inhibitor or
- at least 24 hours after stopping treatment with a reversible MAO-inhibitor (eg moclobemide, linezolid, methylthioninium chloride (methylene blue; this is a reversible non-selective MAO-inhibitor, used as a pre-operative coloring agent).
Initiation of therapy with any MAO inhibitor should occur at least one week after stopping treatment with paroxetine.
Paroxetine should not be used in combination with thioridazine as, as with other CYP450 2D6 hepatic enzyme inhibitors, paroxetine may elevate plasma levels of thioridazine (see 4.5 Interactions with other medicinal products and other forms of interaction).
Administration of thioridazine alone can induce QTc interval prolongation associated with severe ventricular arrhythmias such as torsades de pointes and sudden death.
Paroxetine should not be used in combination with pimozide (see section 4.5 Interactions with other medicinal products and other forms of interaction).
04.4 Special warnings and appropriate precautions for use -
Paroxetine treatment should be initiated with caution two weeks after cessation of irreversible MAO inhibitor treatment or 24 hours after cessation of reversible MAO inhibitor treatment. The dosage of paroxetine should be gradually increased until an optimal response is achieved (see 4.3 Contraindications and 4.5 Interactions with other medicinal products and other forms of interaction).
For use by children and adolescents under the age of 18
Paroxetine should not be used to treat children and adolescents under 18 years of age. In clinical trials, an increase in suicide-related behaviors (suicide attempts and suicidal thoughts) and hostile attitudes (predominantly aggression, oppositional behavior and anger) was observed more frequently in children and adolescents treated with antidepressants compared to those treated with placebo. . If, based on medical needs, a decision to treat is made, the patient should be carefully monitored for the appearance of suicidal symptoms.
Furthermore, there are no long-term safety data in children and adolescents relating to growth, maturation and cognitive and behavioral development.
Suicide / suicidal thoughts or clinical worsening
Depression is associated with an increased risk of suicidal thoughts, self harm and suicide (suicide-related events). This risk persists until significant remission occurs. As improvement may not occur during the first or immediate weeks of treatment, patients should be monitored closely until improvement occurs. It is generally clinical experience that the risk of suicide may increase in the early stages of improvement.
Other psychiatric conditions for which paroxetine is prescribed may also be associated with an increased risk of suicide-related events. Additionally, these conditions can be associated with major depressive disorder. The same precautions followed when treating patients with major depressive disorder should therefore be observed when treating patients with other psychiatric disorders.
Patients with a history of suicide-related events, or who exhibit a significant degree of suicidal ideation prior to initiation of treatment, are at increased risk for suicidal thoughts or suicide attempts, and should be closely monitored during treatment.
A meta-analysis of clinical trials conducted with antidepressant drugs compared to placebo in the treatment of psychiatric disorders in adult patients showed an increased risk of suicidal behavior in the age group below 25 years in patients treated with antidepressants compared to placebo (see Section 5.1).
Drug therapy with antidepressants should always be associated with close surveillance of patients, particularly those at high risk, especially in the initial stages of treatment and after dose changes.
Patients (and caregivers) should be advised of the need to monitor and report immediately to their treating physician any clinical worsening, occurrence of suicidal behavior or thoughts, or changes in behavior.
Akathisia / psychomotor agitation
The use of paroxetine has been associated with the development of akathisia, characterized by an internal feeling of restlessness and psychomotor agitation such as an inability to sit or stand still generally associated with subjective malaise. This is most likely to happen within the first few weeks of treatment. In patients with these symptoms, increasing the dose may be harmful.
Serotonin syndrome / neuroleptic malignant syndrome
On rare occasions, cases suggestive of serotonin syndrome or neuroleptic malignant syndrome have been reported in association with paroxetine treatment, particularly when administered concomitantly with other serotonergic and / or neuroleptic drugs. Since these syndromes can lead to potentially life-threatening conditions, treatment with paroxetine should be discontinued in the event of such events (characterized by symptom pictures such as hyperthermia, rigidity, myoclonus, autonomic imbalances with possible rapid fluctuation of signs mental status changes including confusion, irritability, extreme agitation leading to delirium and coma) and symptomatic supportive treatment should be initiated. Paroxetine should not be used in combination with serotonin precursors (such as L-tryptophan, oxitriptan) due to the risk of serotonin syndrome (see sections 4.3 Contraindications and 4.5 Interactions with other medicinal products and other forms of interaction).
Mania
As with all antidepressants, paroxetine should be used with caution in patients with a history of mania.
Paroxetine should be discontinued in all patients entering a manic phase.
Renal / hepatic insufficiency
Caution is recommended in patients with severe renal insufficiency or in patients with hepatic insufficiency (see section 4.2 Posology and method of administration).
Diabetes
In diabetic patients, treatment with SSRIs can impair glycemic control. The dosage of insulin and / or oral hypoglycaemics may need to be adjusted.
In addition, some studies have suggested that increased blood glucose levels may occur when paroxetine and pravastatin are co-administered (see section 4.5).
Epilepsy
As with other antidepressants, paroxetine should be used with caution in patients with epilepsy.
Convulsions
The overall incidence of seizures in patients treated with paroxetine is less than 0.1%. The drug should be discontinued in all patients who present with seizures.
Electroconvulsive Therapy (ECT)
There is limited clinical experience in the concomitant administration of paroxetine with electroconvulsant therapy (ECT).
Glaucoma
As with other SSRIs, paroxetine can cause mydriasis and should be used with caution in patients with narrow-angle glaucoma or a history of glaucoma.
Cardiovascular pathologies
In patients with cardiovascular diseases the usual precautions should be observed.
Hyponatremia
Hyponatremia has been reported rarely, predominantly in the elderly. Caution should also be exercised in those patients at risk of hyponatremia, for example from concomitant medications and cirrhosis.
Hyponatremia is usually reversible after stopping paroxetine.
Hemorrhages
Cases of cutaneous bleeding disorders such as ecchymosis and purpura have been reported with SSRIs. Other haemorrhagic manifestations, for example gastrointestinal haemorrhages, have been reported.
Elderly patients may be at increased risk.
Caution is advised in patients taking SSRIs concomitantly with oral anticoagulants, drugs known to affect platelet function, or other drugs that may increase the risk of bleeding (e.g. atypical antipsychotics such as clozapine, phenothiazine, most tricyclic antidepressants, acid acetylsalicylic, non-steroidal anti-inflammatory drugs (NSAIDs), COX-2 inhibitors) and in patients with a history of bleeding disorders or conditions that may predispose to bleeding.
Interaction with tamoxifen
Studies have shown that the efficacy of tamoxifen in prophylaxis of the risk of breast cancer recurrence and mortality may be reduced by co-administration with paroxetine, due to an irreversible inhibition of CYP2D6 caused by paroxetine itself (see section 4.5. ).
Where possible, the use of paroxetine should therefore be avoided while using tamoxifen for the treatment or prevention of breast cancer.
Withdrawal symptoms observed on discontinuation of paroxetine treatment
Discontinuation symptoms observed when treatment is stopped are common, particularly in the event of abrupt discontinuation (see section 4.8 Undesirable effects).
In clinical trials, undesirable events observed on discontinuation of treatment occurred in 30% of patients taking paroxetine, compared with 20% of patients taking placebo:
the onset of withdrawal symptoms is not the same in cases where a drug is addictive or addictive.
The risk of withdrawal symptoms may be dependent on several factors, including the duration of therapy, the dose and the rate of dose reduction.
Dizziness, sensory disturbances (including paraesthesia and electric shock sensation and tinnitus), sleep disturbances (including intense dreams), agitation or anxiety, nausea, tremor, confusion, sweating, headache, diarrhea, palpitations, emotional instability, have been reported. irritability and visual disturbances.
Generally the intensity of these symptoms is mild to moderate, however in some patients they may be severe. They generally appear within the first few days of stopping treatment, but there have been very rare cases in which they have occurred in patients who have inadvertently missed a treatment. dose.
Generally these symptoms are self-limiting, and usually resolve within two weeks, although in some individuals they may last longer (2-3 months or more). It is therefore recommended that the dose of paroxetine be gradually reduced when discontinuing treatment over a period of several weeks or months, depending on the patient's needs (see "Withdrawal Symptoms Seen Following Discontinuation of Paroxetine". , section 4.2 Posology and method of administration).
Warnings relating to excipients
Sucrose
The product contains sucrose; therefore patients with rare hereditary problems of fructose intolerance, glucose / galactose malabsorption syndrome or sucrase-isomaltase insufficiency should not take this medicinal product. It can be bad for your teeth.
Ethyl alcohol
The product contains aniseed aroma which is based on ethyl alcohol; the resulting amount of ethyl alcohol in the medicinal product is 26.4 mg / ml, therefore each dose contains an amount of alcohol between 0.0264 g and 0.158 g. This should be taken into account in patients suffering from alcoholism, in pregnant or breastfeeding women, in children and in patients suffering from liver disease or epilepsy.
For those who carry out sporting activities, the use of medicines containing ethyl alcohol can determine positive doping tests in relation to the alcohol concentration limits indicated by some sports federations.
04.5 Interactions with other medicinal products and other forms of interaction -
Pravastatin
Some studies have shown an "interaction between paroxetine and pravastatin, suggesting that co-administration of paroxetine and pravastatin may lead to an increase in blood glucose levels. In patients with diabetes mellitus, receiving both paroxetine and pravastatin, it may be necessary modify the dosage of oral hypoglycemic drugs and / or insulin (see section 4.4).
Serotonergic drugs
As with other SSRIs, co-administration with serotonergic drugs may lead to serotonin-associated effects (serotonin syndrome: see section 4.3 Contraindications and section 4.4 Special warnings and special precautions for use).
Caution should be advised and closer clinical monitoring is required when serotonergic drugs (such as L-tryptophan, triptans, tramadol, linezolid, methylthioninium chloride (methylene blue) SSRIs, lithium and St. John's wort preparations - Hypericum perforatum) they are administered concomitantly with paroxetine.
Caution is also advised with fentanyl, used in general anesthesia or in the treatment of chronic pain.
The concomitant use of paroxetine and MAO inhibitors is contraindicated due to the risk of serotonin syndrome (see section 4.3 Contraindications).
Pimozide
An average 2.5-fold increase in pimozide levels occurred in a low single dose study of pimozide (2 mg) when it was co-administered with paroxetine at a dose of 60 mg. This can be explained on the basis of the inhibitory effect that paroxetine has on CYP2D6. Due to the reduced therapeutic index of pimozide and its known ability to prolong the QT interval, concomitant use of pimozide and paroxetine is contraindicated (see section 4.3 Contraindications).
Enzymes responsible for drug metabolism
The metabolism and pharmacokinetics of paroxetine may be affected by the induction or inhibition of drug metabolizing enzymes.
When paroxetine is administered concomitantly with a drug known to inhibit enzyme metabolism, the use of the lowest doses in the dose range should be considered.
When co-administered with drugs known to induce enzyme metabolism (e.g. carbamazepine, rifampicin, phenobarbital, phenytoin) or with fosamprenavir / ritonavir, no starting dose adjustment is required. Any modification of the paroxetine posology (either after initiation or after discontinuation of an enzyme inducer) should be based on clinical response (tolerability and efficacy).
Fosamprenavir / ritonavir: Co-administration of fosamprenavir / ritonavir 700/100 mg twice daily with paroxetine 20 mg daily in healthy volunteers for 10 days significantly reduces plasma paroxetine levels by approximately 55%. Plasma levels of fosamprenavir / ritonavir during co-administration with paroxetine were similar to reference values from other studies, indicating that paroxetine has no significant effect on the metabolism of fosamprenavir / ritonavir. There are no data on the long-term effect of co-administration of paroxetine and fosamprenavir / ritonavir for longer than 10 days.
Procyclidine: Daily administration of paroxetine significantly increases plasma levels of procyclidine. If anticholinergic effects are observed, the dose of procyclidine should be reduced.
Anticonvulsants: carbamazepine, phenytoin, sodium valproate. Concomitant administration does not appear to show any effect on the pharmacokinetic and pharmacodynamic profile in epileptic patients.
Inhibitory potency of paroxetine on CYP2D6
Like other antidepressants, including other SSRIs, paroxetine inhibits the hepatic cytochrome P450 enzyme CYP2D6. Inhibition of CYP2D6 may lead to increased plasma concentrations of co-administered drugs metabolised by this enzyme. They include these drugs. , some tricyclic antidepressants (e.g. clomipramine, nortriptyline and desipramine), phenothiazine neuroleptics (e.g. perphenazine and thioridazine, see section 4.3 Contraindications), risperidone, atomoxetine, some Type 1 C antiarrhythmics (e.g. propafenone and metoprolainide).
The use of paroxetine in combination with metoprolol administered in heart failure is not recommended due to the reduced therapeutic index of metoprolol in this indication.
Tamoxifen has an important metabolite, endoxifen, which is produced by CYP2D6 and contributes significantly to the efficacy of tamoxifen (see section 4.4).
Irreversible inhibition of CYP2D6 by paroxetine reduces endoxifen concentrations in plasma (see section 4.4).
Alcohol
As with other psychotropic drugs, patients should be advised to avoid alcohol use while taking paroxetine.
Oral anticoagulants
There may be a pharmacodynamic interaction between paroxetine and oral anticoagulants. Concomitant use of paroxetine and oral anticoagulants may lead to an increase in anticoagulant activity and the risk of bleeding. Therefore paroxetine should be used with caution in patients being treated with oral anticoagulants (see section 4.4 Special warnings and special precautions for use. ).
Non-steroidal anti-inflammatory drugs (NSAIDs), acetylsalicylic acid and other antiplatelet agents
A pharmacodynamic interaction between paroxetine and NSAID / acetylsalicylic acid may occur. Concomitant use of paroxetine and NSAIDs / acetylsalicylic acid may lead to an increased risk of bleeding (see section 4.4 Special warnings and special precautions for use).
Caution is advised in patients taking SSRIs concomitantly with oral anticoagulants, drugs known to affect platelet function or other drugs that may increase the risk of bleeding (e.g. atypical antipsychotics such as clozapine, phenothiazine, most tricyclic antidepressants, acetylsalicylic acid , non-steroidal anti-inflammatory drugs (NSAIDs), COX-2 inhibitors) and in patients with a history of bleeding disorders or conditions that may predispose to bleeding.
04.6 Pregnancy and breastfeeding -
Fertility
Animal data have shown that paroxetine can affect sperm quality (see section 5.3). In vitro data on human material show some effect on sperm quality, however, in humans patients treated with SSRIs (including paroxetine) have shown that the effect on sperm quality is reversible. No impact on fertility has been observed so far.
Pregnancy
Some epidemiological studies have indicated an increased risk of congenital malformations, particularly cardiovascular (eg, ventricular and atrial septal defects) associated with paroxetine use during the first trimester of pregnancy. The mechanism is unknown.
The data indicate that the risk of giving birth to a newborn with a cardiovascular defect, following maternal exposure to paroxetine, is less than 2/100, compared to the expected risk of approximately 1/100 for these defects in the general population.
Paroxetine should only be administered during pregnancy when strictly indicated. The physician, at the time of the prescription, will have to evaluate the option of alternative treatments in women who are pregnant or who are planning to become pregnant. Abrupt termination during pregnancy should be avoided (see "Withdrawal symptoms observed following discontinuation of paroxetine treatment", section 4.2 "Posology and method of administration").
Newborns should be observed if maternal use of paroxetine continues into the later stages of pregnancy, particularly in the third trimester.
The following symptoms may occur in newborns following maternal use of paroxetine in later stages of pregnancy: respiratory distress, cyanosis, apnea, convulsions, unstable temperature, difficulty in feeding, vomiting, hypoglycemia, hypertonia, hypotonia, hyperreflexia, tremor, nervousness, irritability, lethargy, constant crying, sleepiness and difficulty falling asleep. These symptoms could be due to either serotonergic effects or withdrawal symptoms.In most cases, complications begin immediately upon delivery or soon after (less than 24 hours).
Epidemiological data have suggested that the use of SSRIs during pregnancy, particularly during late pregnancy, may cause an increased risk of persistent pulmonary hypertension of the newborn (PPHN). The observed risk was approximately 5 in 1000 pregnancies. general population 1 to 2 PPHN cases per 1000 pregnancies occur.
Studies in animals have shown reproductive toxicity but did not indicate direct harmful effects with respect to pregnancy, embryo-fetal development, parturition or postnatal development (see section 5.3 Preclinical safety data).
Feeding time
Small amounts of paroxetine are excreted in breast milk. In published studies, serum concentrations in breastfed infants were undetectable (a sign of drug effects.
Since no effects are anticipated, breastfeeding may be considered.
04.7 Effects on ability to drive and use machines -
Clinical experience has shown that paroxetine therapy is not associated with impaired cognitive or psychomotor functions. However, as with all psychoactive drugs, patients should be advised to exercise caution when driving and operating machinery.
Although paroxetine does not increase the psychic and motor damaging effects induced by alcohol intake, concomitant use of paroxetine and alcohol is not recommended.
04.8 Undesirable effects -
Some of the adverse drug reactions listed below may decrease in intensity and frequency with continued treatment and do not generally lead to discontinuation of therapy. Adverse reactions are listed below by organ, organ / system and by frequency. Frequencies are defined as: very common (≥1 / 10), common (≥1 / 100,
Disorders of the blood and lymphatic system
Uncommon: bleeding disorders, particularly affecting the skin and mucous membranes (mostly ecchymosis).
Very rare: thrombocytopenia.
Disorders of the immune system
Very rare: severe and life-threatening allergic reactions (including anaphylactoid reactions and angioedema).
Endocrine pathologies
Very rare: syndrome of inappropriate antidiuretic hormone secretion (SIADH).
Metabolic and nutritional disorders
Common: increases in cholesterol levels, decreased appetite.
Uncommon: impaired glycemic control has been reported in diabetic patients (see section 4.4).
Rare: hyponatremia.
Hyponatremia has been reported mainly in elderly patients and is sometimes due to the syndrome of inappropriate antidiuretic hormone secretion (SIADH).
Psychiatric disorders
Common: sleepiness, insomnia, agitation, abnormal dreams (including nightmares).
Uncommon: confusion, hallucinations.
Rare: manic reactions, anxiety, depersonalization, panic attacks, akathisia (see section 4.4 Special warnings and special precautions for use).
Frequency not known: aggression, suicidal ideation and suicidal behavior.
Cases of suicidal ideation and suicidal behaviors have been reported during paroxetine therapy or soon after treatment discontinuation (see section 4.4 Special warnings and precautions for use).
These symptoms may be due to the underlying disease.
Cases of aggression have been observed in post-marketing experience
Nervous system disorders
Very common: difficulty concentrating.
Common: dizziness, tremors, headache.
Uncommon: extrapyramidal disorders.
Rare: seizures, restless legs syndrome (RLS).
Very rare: serotonin syndrome (symptoms may include agitation, confusion, diaphoresis, hallucinations, hyperreflexia, myoclonus, chills, tachycardia and tremor).
There have been reports of extrapyramidal disorders, including orofacial dystonia, sometimes in patients already suffering from movement disorders or in patients receiving neuroleptics.
Eye disorders
Common: blurred vision.
Uncommon: mydriasis (see section 4.4).
Very rare: acute glaucoma.
Ear and labyrinth disorders
Frequency not known: tinnitus.
Cardiac pathologies
Uncommon: sinus tachycardia.
Rare: bradycardia.
Vascular pathologies
Uncommon: transient rise or fall in blood pressure, postural hypotension.
Transient increases or decreases in blood pressure have been reported following treatment with paroxetine, usually in patients with pre-existing hypertension or anxiety.
Respiratory, thoracic and mediastinal disorders
Common: yawn.
Gastrointestinal disorders
Very common: nausea.
Common: constipation, diarrhea, vomiting, dry mouth.
Very rare: gastrointestinal bleeding.
Hepatobiliary disorders
Rare: increase in liver enzymes.
Very rare: hepatic events (such as hepatitis, sometimes associated with jaundice and / or liver failure).
Elevations of liver enzymes have been reported. In the post-marketing period, hepatic events (such as hepatitis, sometimes associated with jaundice and / or liver failure) have also been reported very rarely. Discontinuation of treatment should be considered in the event of a prolonged increase. of liver function test values.
Skin and subcutaneous tissue disorders
Common: sweating.
Uncommon: skin rash, pruritus.
Very rare: severe skin adverse reactions (including erythema multiforme, Stevens-Johnson syndrome and toxic epidermal necrolysis), urticaria, photosensitivity reactions.
Renal and urinary disorders
Uncommon: urinary retention, urinary incontinence.
Diseases of the reproductive system and breast
Very common: sexual dysfunction.
Rare: hyperprolactinaemia / galactorrhea.
Very rare: priapism.
Musculoskeletal and connective tissue disorders
Rare: arthralgia, myalgia.
Epidemiological studies, conducted mainly in patients 50 years of age and older, show an increased risk of bone fractures in patients given SSRIs. The factors causing this increased risk are not known.
General disorders and administration site conditions
Common: asthenia, weight gain.
Very rare: peripheral edema.
WITHDRAWAL SYMPTOMS OBSERVED ON DISCONTINUATION OF THE
TREATMENT WITH PAROXETINE
Common: dizziness, sensory disturbances, sleep disturbances, anxiety, headache.
Uncommon: agitation, nausea, tremor, confusion, sweating, emotional instability, visual disturbances, palpitations, diarrhea, irritability.
Discontinuation of paroxetine treatment (especially if abrupt) usually leads to withdrawal symptoms.
Dizziness, sensory disturbances (including paraesthesia and electric shock sensation and tinnitus), sleep disturbances (including intense dreams), agitation or anxiety, nausea, tremor, confusion, sweating, headache, diarrhea, palpitations, emotional instability, have been reported. irritability and visual disturbances.
Generally these events are mild to moderate and self-limiting, however in some patients they may be severe and / or prolonged. It is therefore recommended that, if treatment with paroxetine is no longer required, there is a gradual discontinuation, conducted by a gradual decrease of the dose (see section 4.2 Posology and method of administration and section 4.4 Special warnings and appropriate precautions for use).
ADVERSE EVENTS OBSERVED DURING CLINICAL STUDIES IN PEDIATRIC AGE PATIENTS
The following adverse events were observed:
Increased suicide-related behaviors (including suicide attempts and suicidal ideation), self-harming behavior and increased hostile attitude. Suicidal thoughts and suicide attempts were mainly observed in clinical trials with adolescents with Major Depressive Disorder. hostile attitude has particularly occurred in children with OCD and especially in children under the age of 12.
Additional events that have been observed are: decreased appetite, tremor, sweating, hyperkinesis, agitation, emotional lability (including crying and mood fluctuations), haemorrhagic adverse events, especially affecting the skin and mucous membranes.
Events observed following withdrawal / tapering of paroxetine are: emotional lability (including crying, mood fluctuations, self harm, suicidal thoughts and suicide attempts), nervousness, dizziness, nausea and abdominal pain (see section 4.4 Special and appropriate warnings precautions for use).
See section 5.1 for more information on pediatric clinical trials.
Reporting of suspected adverse reactions
Reporting of suspected adverse reactions occurring after authorization of the medicinal product is important as it allows continuous monitoring of the benefit / risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system. "address: www.agenziafarmaco.gov.it/it/responsabili.
04.9 Overdose -
Symptoms and signs
Based on the available information regarding overdose with paroxetine, a large margin of safety appears evident.
Experience with paroxetine overdose has indicated that, in addition to the symptoms described in section 4.8 Undesirable effects, fever and involuntary muscle contractions have been reported.
Patients generally recovered without serious sequelae even in cases where paroxetine was taken alone up to doses of 2000 mg. Events such as coma or ECG changes have occasionally been reported, very rarely with a fatal outcome, but generally when paroxetine was taken in combination with other psychotropic drugs, with or without alcohol.
Treatment
No specific antidote is known.
Treatment should be based on the general measures used in the treatment of overdose with antidepressants. To reduce the absorption of paroxetine, administration of 20-30 g of activated charcoal may be considered, if possible within hours of taking the overdose. Supportive therapy with careful observation and frequent monitoring of vital signs is indicated. Patient management should follow clinical indications.
05.0 PHARMACOLOGICAL PROPERTIES -
05.1 "Pharmacodynamic properties -
Pharmacotherapeutic group: antidepressants - selective serotonin reuptake inhibitors.
ATC code: N06A B05.
Mechanism of action
Paroxetine is a potent and selective 5-hydroxytryptamine (5-HT; serotonin) reuptake inhibitor; its antidepressant action and its efficacy in the treatment of obsessive compulsive disorder, social anxiety disorder / social phobia, generalized anxiety disorder, post-traumatic stress disorder and panic disorder are believed to be related to this specific inhibition of reuptake of 5-HT in brain neurons.
Paroxetine is not chemically related to tricyclics, tetracyclics and other available antidepressants.
Paroxetine has low affinity for muscarinic-type cholinergic receptors and studies in animals have shown only weak anticholinergic properties.
In agreement with this selectivity of action, some studies in vitro showed that, unlike tricyclic antidepressants, paroxetine has low affinity for alpha 1, alpha 2 and beta-adrenoceptors, for dopamine receptors (D2), for 5-HT1 like and 5-HT2 receptors, and for those of "histamine (H1).
This lack of interaction with postsynaptic receptors in vitro has been confirmed by studies in vivo, which demonstrated the absence of depressive properties on the central nervous system and of hypotensive properties.
Pharmacodynamic effects
Paroxetine does not alter psychomotor functions and does not potentiate the depressive effects of ethanol.
Similar to other selective serotonin reuptake inhibitors, paroxetine causes symptoms related to excessive stimulation of the serotonin receptor when administered to animals previously treated with monoamine oxidase (MAO) inhibitors or tryptophan.
Behavioral and EEG studies indicate that paroxetine is weakly activating at doses generally higher than those required to inhibit serotonin reuptake. The activating properties are not by nature "amphetamine-like". Animal studies indicate that paroxetine is well tolerated by the cardiovascular system. Paxoxetine does not cause significant changes in blood pressure, heart rate and ECG after administration to healthy subjects.
Studies indicate that paroxetine, unlike antidepressants which inhibit noradrenaline reuptake, has a more reduced propensity to inhibit the antihypertensive effects of guanethidine.
Paroxetine, in the treatment of depressive disorders, demonstrates efficacy comparable to that of standard antidepressants.
There is also some evidence that paroxetine may have therapeutic value in patients who are unresponsive to standard therapy.
Administration of the dose in the morning has no adverse effect on the quality or duration of sleep. Additionally, patients may report improved sleep when they respond to paroxetine therapy.
Analysis of the risk of suicide in adults
A paroxetine-specific analysis of clinical trials conducted in comparison with placebo in adult patients with psychiatric disorders showed a higher frequency of suicidal behavior in young adults (aged 18 to 24 years) treated with paroxetine compared to placebo ( 2.19% compared to 0.92%). In the older age groups, no such increase was observed. In adults (of all ages) with major depressive disorder, there was an increased frequency of suicidal behavior in patients treated with paroxetine compared to placebo (0.32% compared to 0.05%); all events were suicide attempts. However, the majority of such attempts for paroxetine (8 of 11) occurred in young adults (see also section 4.4).
Dose response
In fixed dose studies, the dose response curve is flat, indicating no efficacy advantage in using higher than recommended doses. However, there are some clinical data that suggest that subsequent dose increases may be of benefit to some. patients.
Long-term efficacy
The long-term efficacy of paroxetine in depression was demonstrated in a 52-week maintenance study designed to evaluate relapse prevention: relapses in patients treated with paroxetine (20-40 mg per day) occurred in the 12% of cases, compared to 28% of cases in patients taking placebo.
The long-term efficacy of paroxetine in the treatment of OCD was examined in three 24-week maintenance studies, designed to evaluate relapse prevention. In one of the three studies, a significant difference was achieved in the proportion of patients with relapses between paroxetine (38%) and placebo (59%).
The long-term efficacy of paroxetine in the treatment of panic disorder was demonstrated in a 24-week maintenance study designed to evaluate relapse prevention: relapses in patients treated with paroxetine (10-40 mg per day) occurred in 5% of cases, compared to 30% of patients taking placebo.This was supported by a 36-week maintenance study.
The long-term efficacy of paroxetine in the treatment of social and generalized anxiety disorders and post-traumatic stress disorder has not been sufficiently demonstrated.
Adverse Events Observed in Clinical Trials in Pediatric Patients
During short-term clinical studies (up to 10-12 weeks) in children and adolescents, the following adverse events have been reported in patients treated with paroxetine with a frequency of at least 2% of patients and with at least double the incidence compared to placebo: increased suicide-related behaviors (including suicide attempts and suicidal thoughts), self-harming behavior and increased hostile attitude.
Suicidal thoughts and suicide attempts were mainly observed in clinical trials in adolescents with Major Depressive Disorder. The increase in hostile attitude has particularly occurred in children with OCD, especially in children under the age of 12. Additional events that were observed more frequently in the paroxetine group than in the placebo group were: decreased appetite, tremor, sweating, hyperkinesis, agitation, emotional lability (including crying and mood fluctuations).
In studies where the tapering regimen was used, symptoms reported during the tapering phase or upon discontinuation of paroxetine, observed with a frequency of at least 2% of patients and occurring occurred with at least double the incidence of placebo, were: emotional lability (including crying, mood swings, self harm, suicidal thoughts and suicide attempts), nervousness, dizziness, nausea and abdominal pain (see section 4.4 Special and appropriate warnings precautions for use).
In five parallel group studies lasting from eight weeks to eight months, bleeding-related adverse events, mainly of the skin and mucous membranes, were observed in patients treated with paroxetine with a frequency of 1.74% of the frequency of 0. , 74% observed in patients treated with placebo.
05.2 "Pharmacokinetic properties -
Absorption
Paroxetine is well absorbed after oral administration and undergoes first pass metabolism.
Due to first pass metabolism, the amount of paroxetine available in the systemic circulation is less than that absorbed from the gastrointestinal tract. In case of increased body burden following higher single doses or multiple doses, partial saturation of the first pass effect and a reduction in plasma clearance occur. This leads to a disproportionate increase in plasma concentrations of paroxetine and therefore pharmacokinetic parameters are not constant, resulting in non-linear kinetics, however non-linearity is generally modest and is limited to those subjects who achieve low plasma levels at low doses.
Systemic steady-state levels are achieved within 7-14 days of initiation of treatment with the immediate or controlled release formulations and pharmacokinetics do not appear to change during long-term treatment.
Distribution
Paroxetine is widely distributed in tissues and pharmacokinetic calculations indicate that only 1% of the paroxetine present in the body is found in the plasma. About 95% of the paroxetine present in plasma is bound to proteins at therapeutic concentrations.
No correlation has been demonstrated between paroxetine plasma concentrations and clinical effects (adverse events and efficacy).
The passage into human breast milk, and into the fetuses of laboratory animals, occurs in small quantities.
Metabolism
The major metabolites of paroxetine are polar and conjugated products of oxidation and methylation, which are readily cleared. In view of their relative lack of pharmacological activity, they are extremely unlikely to contribute to the therapeutic effects of paroxetine.
Metabolism does not compromise the selectivity of action of paroxetine on neuronal reuptake of serotonin.
Elimination
Urinary excretion of unchanged paroxetine is generally less than 2%, while that of metabolites is about 64% of the dose. Approximately 36% of the dose is excreted in the faeces, probably via bile, of which unchanged paroxetine represents less than "1% of the dose. Thus paroxetine is almost completely eliminated by metabolism.
Excretion of metabolites is biphasic, being initially the result of first pass metabolism and subsequently controlled by the systemic elimination of paroxetine.
The elimination half-life is variable but is generally about one day.
Special patient populations
Elderly and renal / hepatic insufficiency
An increase in plasma concentrations of paroxetine has been observed in elderly subjects and in subjects with severe renal insufficiency and in subjects with hepatic insufficiency, but the range of plasma concentrations is similar to that of healthy adult subjects.
05.3 Preclinical safety data -
Toxicological studies were conducted in the rhesus monkey and in the albino rat; in both species the metabolic profile is similar to that described in humans. As expected with lipophilic amines, including tricyclic antidepressants, phospholipidosis was detected in rats. Phospholipidosis was not observed in primate studies, lasting up to a year, at doses six times higher than the recommended clinical dose range.
Carcinogenicity: In two-year studies conducted in mice and rats, paroxetine did not show carcinogenic effects.
Genotoxicity: Genotoxicity was not observed in a series of tests in vitro And in vivo.
Reproductive toxicity studies in rats showed that paroxetine affects male and female fertility by reducing the fertility index and pregnancy rate. In rats, higher infant mortality and delayed ossification were observed. The latter effects are likely related to maternal toxicity and are not considered to be a direct effect on the fetus / neonate.
06.0 PHARMACEUTICAL INFORMATION -
06.1 Excipients -
Hydroxypropylbetadex
Sucrose
Anise flavor (anethole, water, ethyl alcohol)
Sodium benzoate E 211
Purified water
1N hydrochloric acid
06.2 Incompatibility "-
None.
06.3 Period of validity "-
3 years in the original unopened container.
30 days after first opening the 30 ml bottle.
60 days after first opening the 60 ml bottle.
06.4 Special precautions for storage -
This medicinal product does not require any special storage conditions.
06.5 Nature of the immediate packaging and contents of the package -
Amber glass bottle containing 30 ml or 60 ml of solution, closed with a white aluminum screw cap. A glass dropper with child-proof polypropylene cap is attached to the bottle.
06.6 Instructions for use and handling -
No special instructions.
07.0 HOLDER OF THE "MARKETING AUTHORIZATION" -
Lifepharma S.p.A - Via dei Lavoratori, 54 - 20092 Cinisello balsamo (MI)
Dealer for sale
Polifarma S.p.A - Viale dell "Arte 69 - 00144 Rome
08.0 MARKETING AUTHORIZATION NUMBER -
STILIDEN 10 mg / ml oral drops, solution - 30 ml bottle AIC: 036451019
STILIDEN 10 mg / ml oral drops, solution - 60 ml bottle AIC: 036451058
09.0 DATE OF FIRST AUTHORIZATION OR RENEWAL OF THE AUTHORIZATION -
Authorization: March 2006
Renewal: February 2011
10.0 DATE OF REVISION OF THE TEXT -
May 2015















-nelle-carni-di-maiale.jpg)