Absorption of iron
The iron present in the body derives from the dietary intake, which allows to maintain a balance between absorption and daily losses.

A "common" diet involves the intake of 10-20 mg of iron per day, but under normal conditions only 5-10% (about 1-2 mg) is absorbed. If the requirement is increased it can even reach 20. -30%.
Absorption regulation
The maintenance of homeostasis (balance between gains and losses) of iron is ensured by the regulation of intestinal absorption, which is increased for the needs of erythropoiesis and reduced when iron deposits are abundant.
Foods rich in iron are liver, red meats, oysters and legumes.
Its absorption is reduced in cases of:
- Low iron diet (in absolute terms, but increases in percentage terms)
- Alterations in gastric pH: a reduction in gastric acidity reduces its absorption
- Chelating agents in the diet: substances that bind it, reducing the amount available
- The possible decrease of absorbent intestinal surface or the alterations of the absorbent cells that constitute it
- Situations of increased intestinal motility
- Hemochromatosis (hereditary disease)
- Situations that increase iron turnover, such as vitamin B12 deficiency (pernicious or nutritional deficiency) or folate anemia
- Metabolic disorders
- Presence in food of EDTA (a preservative), of Tannates (substances present in tea), of oxalates, phosphates and carbonates.
On the other hand, ascorbic acid (vitamin C), citric acid, amino acids and sugars of food origin facilitate its absorption.
Iron is absorbed as heme iron, that is bound to hemoglobin or myoglobin present in meat. Or it can be absorbed in soluble form (ferrous). The iron in heme is much more absorbable than inorganic.
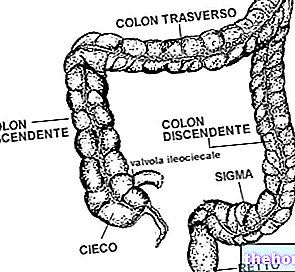
Absorption occurs in the duodenum (the first portion of the small intestine) and in the first part of the jejunum (intermediate portion of the small intestine).
The body regulates the amount of iron to be absorbed with three mechanisms:
- Through a deposit regulator that signals the depletion status of the deposits themselves.
- By means of an erythropoiesis regulator, which signals the amount of iron available for the synthesis of erythrocytes.
- By means of a mechanism in the kidney that signals the degree of hypoxia.
Iron in the blood
Once absorbed in the intestine, iron enters the bloodstream bound to a protein called transferrin, and here it is found in a closed system where it is constantly recycled between plasma and tissues.
In clinical practice it is very useful to dose:
The amount of circulating transferrin saturated in iron, a value that takes the name of sideremia, and whose normal values are between 15 and 120 milligrams per deciliter.
The total iron binding capacity, which is called transferrinemia, and whose normal values are between 250 and 400 milligrams per deciliter.
Transferrin plays a key role in hematopoiesis, as it is responsible for the transfer of iron to erythroblasts, which have a specific receptor for it on their surface.
Iron losses
The physiological excretion of iron occurs with urine, faeces, sweat, desquamation of intestinal cells, skin, urinary tract. Iron losses in men and women after menopause amount to about 1 mg per day . In women of childbearing age, losses are increased in consideration of the menstrual cycle (usually up to about 25 mg / cycle) and pregnancies, since, from conception to childbirth, there is an additional loss of iron of about 700 mg, if they consider the shares given to the fetus, the expulsion of the placenta and the post-partum haemorrhage; the loss due to breastfeeding is approximately 1 mg per day.
Iron metabolism
Under normal conditions, the iron content of the whole organism ranges from 2g in women up to 6g in men. The iron is divided into a functional compartment and a storage compartment. About 80% of functional iron is found in hemoglobin, myoglobin and iron-containing enzymes. About 15% of the total iron is found in the storage pool, consisting of hemosiderin and ferritin. It should be noted that young women, even in good health, have significantly lower iron deposits than men. Their martial balance (of iron) is therefore much more precarious and they are consequently more vulnerable to excessive losses or increased demands related to the menstrual cycle and pregnancy.
All the storage iron is accumulated in the form of ferritin or hemosiderin. Ferritin is essentially an iron-protein complex found in all tissues, but particularly in the liver, spleen, bone marrow and skeletal muscles.
When iron deposits are normal, only traces of hemosiderin are found in the body. It is made up of aggregates of ferritin molecules. Under martial overload conditions, most of the iron is deposited in the form of hemosiderin.
Normally very small amounts of ferritin circulate in the plasma. Plasma ferritin derives largely from the deposit pool and therefore its dosage is a good indicator of the adequacy of the organism's martial reserves. In deficient situations, serum ferritin is always less than 12 micrograms per liter while in overload conditions very high values can also be found, close to 5 thousand micrograms per liter.
The physiological importance of the martial reserve pool is the ease of mobilization in the event of an increase in demands.
Under normal conditions, there is a balance between the amount of ferritin in the deposits and that in the plasma. This is a useful parameter for evaluating the body's martial reserves.
There are some situations in which iron deposits grow:
In case of overload deriving from a high iron intake, as for example in subjects who need continuous blood transfusions or in those suffering from a genetic disease called hemosiderosis.
In chronic inflammatory or tumor processes, in which iron is brought from the circulating (usable) compartment to that of deposits, with a consequent picture of chronic disease anemia, characterized by a reduction in circulating iron (hyposideremia) and an increase in that deposit (hyperferritinemia).
Important destruction of the tissues: they lead to a release into the circulation of the iron contained in the damaged cells with a consequent increase in circulating ferritin.