Biological role
In the red blood cells of the fetus, it is possible to identify a form of hemoglobin other than the adult one.
- From a structural point of view, fetal hemoglobin (α2γ2) differs from adult hemoglobin (α2Β2) due to the presence of two gamma chains instead of the two alpha chains
- In particular, fetal hemoglobin is made up of two α chains and two γ chains, made up respectively of 141 and 146 amino acids. The two alpha chains are identical to those present in adult hemoglobin, while the gamma ones differ from Beta for 39 amino acids.
- This structural change gives fetal hemoglobin a "affinity for" higher oxygen; in other words, it binds to oxygen more tenaciously than adult hemoglobin
From a functional point of view, fetal hemoglobin (HbF or hemoglobin F.) allows the fetus to more effectively extract oxygen from the maternal blood.
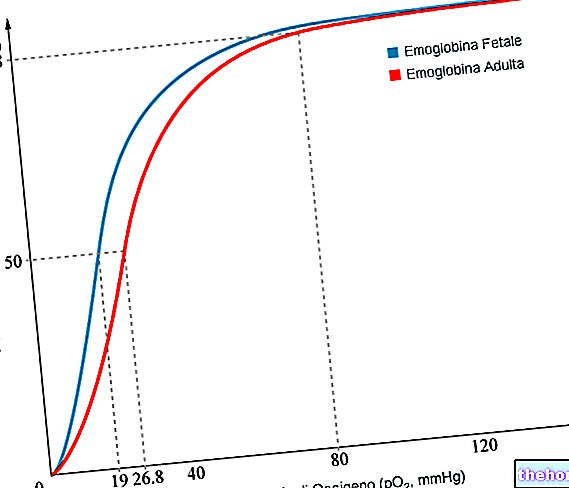
- due to the low PO2 value of fetal blood, fetal hemoglobin can carry up to 20-30% more oxygen than maternal, as shown by the graph below; this is due to the lower affinity for 2,3-bisphosphoglycerate compared to to adult hemoglobin

We observe the shift to the left of the dissociation curve of fetal hemoglobin with respect to the adult one
The transfer of oxygen to fetal blood through the placental barrier is also favored by the higher concentration of hemoglobin, which is approximately 50% higher than that of maternal blood.
Normal Values
The synthesis of fetal hemoglobin begins around the sixth week of gestation, and gradually replaces the embryonic hemoglobins Gower (ζ2ε2), Gower II (α2ε2), and Portland (ζ2γ2), produced in the first weeks of conception.

Expression over time and in different tissues of the different types of human globin chains.
Human globin chains are expressed as a percentage of hemoglobin over the total
The synthesis of beta globins characterizing adult hemoglobin, barely perceptible during fetal life, reaches the normal regime only towards the end of the third month of extrauterine life.
- at birth, fetal hemoglobin constitutes about 70-90% of the total hemoglobin present in the red blood cells of the newborn
- the synthesis of fetal hemoglobin continues even after birth, but gradually decreases, making up less than 8% of all hemoglobin at the stroke of six months of life
- within the first year of life, fetal hemoglobin concentrations drop to levels generally below 1%
- Normal adults have fetal hemoglobin values between 0.3% and 1.2%, less than 3.5% hemoglobin A2 (α2, δ2), and the remaining percentage (usually> 96%) covered by hemoglobin type A.
The different expression over time, from conception to adult life, of the different globin chains in man depends on the activation and deactivation of specific genes.
High Fetal Hemoglobin
Pathological significance
- In the uterus, the normal fetus produces a small amount of adult hemoglobin (2.5-5%). The fetus with thalassemia major produces even less (less than 2%). To detect during pregnancy whether a fetus has thalassemia maior, it is possible to determine the amount of adult hemoglobin present in a blood sample taken by cordocentesis.
- A small percentage of fetal hemoglobin is also expressed during adult life and its levels can vary greatly under the influence of factors such as age, sex or genomic peculiarities. Some subjects are affected by the so-called hereditary persistence of fetal hemoglobin, a benign condition in which important concentrations of fetal hemoglobin (> 10%) persist even in adulthood. It has been noted that this peculiarity, generally asymptomatic, can alleviate the severity of certain hemoglobinopathies and thalassemias.
- A drug therapy capable of increasing the concentration of fetal hemoglobin brings significant benefits to some categories of patients, such as those suffering from sickle cell anemia or Beta thalassemia. The prototype of these drugs was hydroxyurea, an antineoplastic drug with myelosuppressive action, which has been shown to be effective in increasing fetal hemoglobin levels and reducing the incidence of painful crises in patients with sickle cell disease.