What is Tamiflu?
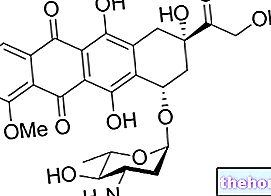
Tamiflu is a medicine that contains the active substance oseltamivir.It is available as capsules (yellow: 30 mg; gray: 45 mg; yellow and gray: 75 mg) and as a powder for oral suspension (12 mg / ml).
What is Tamiflu used for?
Tamiflu is used in the treatment or prevention of influenza in patients over one year of age:
- in the treatment of influenza, it can be used in patients who have symptoms of influenza, when the influenza virus is known to be circulating in the community; in the prevention of influenza, it can be used in patients who have been in contact with people affected. This is generally done on a case-by-case basis, but in exceptional circumstances, for example when the flu vaccine is insufficient and in the presence of a pandemic (global flu epidemic), seasonal prevention may be considered.
During an influenza pandemic, Tamiflu can also be used to treat or prevent influenza in children under one year of age. It is up to doctors to decide whether to give Tamiflu to children in this age group, depending on the severity of the disease triggered by the flu virus as well as the health of the child, so as to ensure that the child is likely to benefit from the medicine
Tamiflu is not a substitute for flu vaccination, and its use must be based on official recommendations.
The medicine can only be obtained with a prescription.
How is Tamiflu used?
In the treatment of influenza, Tamiflu should be given within the first two days after symptoms appear. The medicine is taken in a single dose twice a day for five days.
In the prevention of influenza, Tamiflu should be administered within the first two days of contact with people with the flu. The medicine should be taken in a single dose once a day for at least 10 days after contact with an infected individual. In the case of use of Tamiflu during a "flu epidemic, the dose can be given for up to six weeks."
The dose of Tamiflu for adults and children weighing more than 40 kg is 75 mg. For children weighing less than 40 kg, the dose is adjusted to their weight using lower strength capsules
(30 or 45 mg) or oral suspension. If the powder for oral suspension is not available, the pharmacist can prepare a solution using the contents of the capsules, or the contents of the capsules can be mixed at home with sweetened food. In patients with kidney problems it is necessary to reduce the doses. For all information, see the package leaflet.
How does Tamiflu work?
The active substance in Tamiflu, oseltamivir, acts specifically on the influenza virus by blocking some of the enzymes on its surface, known as neuraminidases. When these enzymes are blocked, the virus cannot spread. Oseltamivir acts on the neuraminidase enzymes of viruses. influenza A (the most common) and B.
How has Tamiflu been studied?
Tamiflu has been compared with placebo (a dummy treatment) in influenza treatment studies (involving 2,413 adults and adolescents, 741 elderly and 1,033 children aged at least one year). Efficacy was measured using a test card. evaluation in which symptoms (fever, muscle aches, headache, sore throat, cough, general malaise and runny nose) should be noted.
In the prevention of influenza, Tamiflu has been studied in patients who had been exposed to the disease because a family member contracted the virus (962 cases) or during an epidemic (1 562 subjects aged 16 to 65 and 548 elderly people in retirement homes). Studies recorded the number of influenza cases demonstrated by laboratory tests. A study also investigated the use of Tamiflu in the family setting (277 families) both for the treatment of the affected person and for the treatment or prevention of influenza in people in contact with the patient.
A small study was conducted to show that the recommended dose of Tamiflu produces similar levels of the medicine in the blood of children aged one month and one year to the effective doses in older children and adults. Tamiflu has not been studied in children under one month of age; however its use in this age group is based on the results observed in older children.
What benefit has Tamiflu shown during the studies?
In adult treatment studies, Tamiflu shortened the course of the disease from an average of 5.2 days in patients treated with placebo to 4.2 days in patients treated with Tamiflu. The mean reduction in disease duration in children aged one to six was 1.5 days.
In prevention studies, Tamiflu reduced the incidence of influenza among people in contact with sick people. In the study conducted during an epidemic, only 1% of the people who took Tamiflu developed flu after contact, compared with 5% of the subjects treated with placebo. In families with one member affected by the flu. 7% of cohabiting family members who had taken Tamiflu for preventive purposes developed the disease, compared to 20% of those who had not undergone any preventive treatment.
What is the risk associated with Tamiflu?
The most common side effects of Tamiflu in patients at least 13 years of age (seen in more than 1 in 10 patients) are headache and nausea. In children aged one year to 12 years, the most common side effects (seen in more than 1 in 10 patients) are vomiting and diarrhea; similar side effects are observed in children less than one year of age. For the full list of side effects reported with Tamiflu, see the package leaflet.
Tamiflu must not be used in people who are potentially hypersensitive (allergic) to oseltamivir or any of the other ingredients.
Why has Tamiflu been approved?
The Committee for Medicinal Products for Human Use (CHMP) decided that Tamiflu's benefits are greater than its risks in the treatment and prevention of influenza. The Committee therefore recommended that Tamiflu be given marketing authorization.
Other information about Tamiflu:
On June 20, 2002, the European Commission granted Roche Registration Limited a "Marketing Authorization" for Tamiflu, valid throughout the European Union.. After five years, the marketing authorization was renewed for another five years.
For the full version of Tamiflu's EPAR click here.
Last update of this summary: 10-2009.
The information on Tamiflu - oseltamivir published on this page may be out of date or incomplete. For a correct use of this information, see the Disclaimer and useful information page.