What is Jetrea and what is it used for?
Jetrea is a medicine that contains the active substance ocriplasmin. It is indicated in adults for the treatment of vitreomacular traction (VMT), an eye disease that can cause severe visual disturbances.
How is Jetrea used - Ocriplasmina?
Jetrea is a concentrate to be made up into a solution for ocular injection.The medicine can only be obtained with a prescription and must be prepared and given by an ophthalmologist (specialist in eye diseases) with experience in intravitreal injections (injections into the vitreous body, the gelatinous mass at the back of the eye). . The concentrate must be stored in the freezer and the procedure must take place under sterile conditions.
The recommended dose is 0.125 mg given by injection into the affected eye only once as a single dose. The other eye should not be treated with Jetrea for at least 7 days.
The ophthalmologist may prescribe antibiotic eye drops before and after treatment with Jetrea to prevent eye infections. Patients should be monitored after the injection to rule out any infection or increased intraocular pressure.
How does Jetrea - Ocriplasmina work?
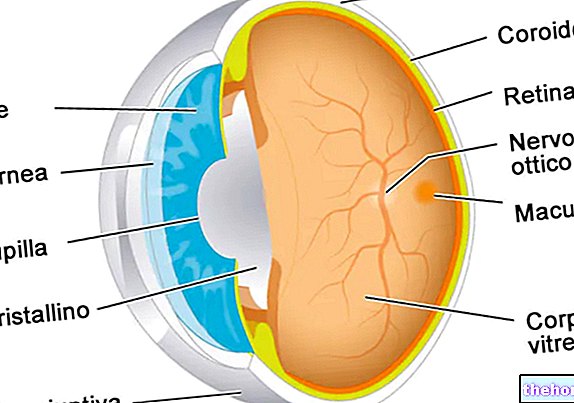
Vitreomacular traction is caused by vitreomacular adhesion (VMA), whereby the vitreous body remains attached with anomalous force to the central part of the retina (the innermost membrane of the eye, sensitive to light). Over the years the size of the body vitreous shrinkage and the area affected by the abnormal attachment can exert traction on the retina, which in turn causes swelling of the retina itself and symptoms such as blurring or distortion of vision.
The active ingredient in Jetrea, ocriplasmin, is similar to human plasmin, an enzyme naturally present in the eye capable of breaking down the proteins responsible for adhesion between the vitreous body and the retina, thereby reducing swelling and improving vision.
The ocriplasmin in Jetrea is produced by a method known as 'recombinant DNA technology': it is made by yeast cells that have received a gene (DNA) that allows them to produce ocriplasmin.
What benefit has Jetrea - Ocriplasmin shown during the studies?
During the studies Jetrea has been shown to effectively resolve the adhesion between the vitreous body and the retina, reducing the need for surgery.
In two main studies involving 652 adults with VMA and decreased vision, patients were given a single dose of 0.125 mg of Jetrea or placebo (a dummy treatment) by intravitreal injection.
After 28 days, adhesions had been cured in 25% and 28% of patients treated with an injection of Jetrea (61 out of 219 and 62 out of 245) compared to 13% and 6% of subjects treated with placebo (14 out of 107 and 5 of 81) Effective treatment of VMA can help resolve vision disturbances caused by VMT and prevent further vision loss associated with progressive, untreated retinal traction.
What are the risks associated with Jetrea - Ocriplasmina?
Side effects seen with Jetrea affect the eye. The most common side effects of Jetrea are flying flies (dark and often irregularly shaped spots in the field of vision), eye pain and photopsia (light sensation in the form of flashes in the field of vision) as well as conjunctival haemorrhage (bleeding from the membrane covering the eyeball). For the full list of side effects reported with Jetrea, see the package leaflet.
Jetrea must not be used in patients with a known or suspected infection in or around the eye. For the full list of restrictions, see the package leaflet.
Why has Jetrea - Ocriplasmina been approved?
The Agency's Committee for Medicinal Products for Human Use (CHMP) concluded that Jetrea's benefits are greater than its risks and recommended that it be approved for use in the EU. Studies have shown that Jetrea was effective in treating vitreomacular adhesion and, therefore, the medicinal product is expected to be effective in preventing episodes of visual impairment, which may occur in the presence of untreated and progressive vitreomacular traction. Although modest (resolution of VMA in a quarter of patients), the benefits of the medicine were considered significant as treatment with Jetrea can improve vision and avoid surgery. As for the safety of the medicine, the most common side effects, which have been short-lived and are considered manageable, are often secondary to the injection procedure or related to the resolution of the disease itself. The risk of more serious side effects, such as irreversible decrease in vision, other alterations in the function of the retina or the supporting structures of the lens, appears reduced.
What measures are being taken to ensure the safe and effective use of Jetrea - Ocriplasmin?
A risk management plan has been developed to ensure that Jetrea is used as safely as possible. Based on this plan, safety information has been added to the summary of product characteristics and package leaflet for Jetrea, including the appropriate precautions to be followed by healthcare professionals and patients.
In addition, the company that makes Jetrea must ensure that all healthcare professionals who may use Jetrea receive an information pack containing essential information on how to use the medicine as well as an information pack to pass on to patients.
Other information about Jetrea - Ocriplasmina
On March 13, 2013, the European Commission issued a "marketing authorization" for Jetrea, valid throughout the European Union.
For more information on Jetrea therapy, read the package leaflet (included with the EPAR) or contact your doctor or pharmacist.
Last update of this summary: 03-2013.
The information on Jetrea - Ocriplasmina published on this page may be out of date or incomplete. For a correct use of this information, see the Disclaimer and useful information page.