ETHINYLESTRADIOL Amsa ® is a drug based on Ethinylestradiol
THERAPEUTIC GROUP: Unassociated semi-synthetic estrogens

Indications ETHINYLESTRADIOL Amsa ® - Ethinylestradiol
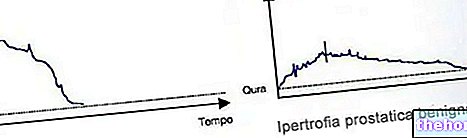
ETINYLESTRADIOL Amsa ® is used in the management of estrogen deficiency symptoms typical of menopause, in the prevention of postmenopausal osteoporosis, in the prevention of lactation and in men in the treatment of prostate disorders.
Mechanism of action ETHINYL ESTRADIOL Amsa ® - Ethinylestradiol
Ethinylestradiol, active principle of ETHINYLESTRADIOL Amsa ® is a semisynthetic estrogen, which shares the same biological activities with estradiol, distinguishing itself instead for its different pharmacokinetic characteristics.
Taken orally, it is rapidly and almost totally absorbed in the intestine, and bound to plasma proteins, mainly albumin, is distributed to various tissues and subsequently metabolized in the liver.
Its metabolites are eliminated both via the bile and the urine at a ratio of 6: 4 after a half-life of approximately 3-24 hours.
Its biological action, carried out through the interaction with nuclear receptors and the modulation of gene expression, materializes in the reduction of post-climacteric symptoms such as: vasomotor disorders and nervous disorders, associated with the estrogen deficiency typical of menopause.
Therapy with estrogens and analogues has also proved particularly effective in the prevention of chronic and degenerative pathologies such as osteoporosis, preserving bone health by balancing the resorption-osteodeposition processes.
Studies carried out and clinical efficacy
1. ETHINYLESTRADIOL AND PSA
Anticancer Res. 2010 Dec; 30: 5201-5.
Ethinylestradiol improves prostate-specific antigen levels in pretreated castration-resistant prostate cancer patients.
Izumi K, Kadono Y, Shima T, Konaka H, Mizokami A, Koh E, Namiki M.
Treatment with oral ethinylestradiol was found to be useful in decreasing blood PSA concentrations by more than 50% in 70% of treated patients. However, side effects include anorexia, liver damage and heart failure.
2.ETINYL ESTRADIOL AND ARGININE
Gynecol Endocrinol. 2010 Dec; 26: 861-8. Epub 2010 Jul 20.
L-arginine plus drospirenone-ethinyl estradiol in the treatment of patients with PCOS: a prospective, placebo controlled, randomized, pilot study.
Battaglia C, Mancini F, Battaglia B, Facchinetti F, Artini PG, Venturoli S.
The addition of arginine to oral ethinylestradiol therapy reduced the incidence of cardiovascular side effects as measured by the evaluation of biochemical and functional parameters, such as Doppler ultrasound, blood pressure monitoring and evaluation of vascular flow.
3. ETHINYLESTRADIOL AND PHYTOESTROGENS
Shokuhin Eiseigaku Zasshi. 2010; 51: 101-9.
Combined estrogenic activity of soybean extract used in a dietary supplement and ethinyl estradiol.
Kawazoe S, Naka K, Ishibashi H, Obara T, Arizono K, Hashimoto K, Hojo Y, Suzuki T.
As it is known, soy is rich in phytoestrogens and is often recommended in the postclimatic period. In this study it was observed how the "addition of this legume to estrogen therapy with ethinyl estradiol can significantly improve the activation status of estrogen receptors, increasing the" therapeutic effect of hormone replacement therapy.
Method of use and dosage
ETHINYLESTRADIOL Amsa ® 10, 50 and 100 mcg ethinyl estradiol tablets:
the dosages of ethinylestradiol to be used in the treatment of post-climacteric symptoms vary from patient to patient according to the clinical condition.
The dosage, defined by the doctor after a "careful clinical-medical examination, may be subject to variations in the course of work" to maximize the therapeutic effects while avoiding the appearance of side effects.
Treatment with ETINYLESTRADIOL Amsa ® generally set according to cyclic protocols must generally be followed by progestogen therapy according to medical indication.
Warnings ETHINYL ESTRADIOL Amsa ® - Ethinylestradiol
The biological complexity of hormone replacement therapy with estrogen requires a particularly careful medical examination before embarking on the therapeutic process.
Medical supervision throughout the treatment is necessary to minimize the possible occurrence of side effects while maintaining high therapeutic efficacy.
Endometriosis, thromboembolic or estrogen-dependent diseases leiomyoma, hypertension, liver disease, diabetes, migraine, cardiovascular pathologies, liver and kidney pathologies, bronchial asthma and allergic pathologies represent only some of the conditions for which particular care must be given, during therapy with estrogen and analogues.
The correct doctor / patient relationship is the basis of therapeutic success and useful for better understanding the potential risks and benefits of therapy, helping the patient to promptly recognize the first side effects by applying useful and effective remedies.
PREGNANCY AND BREASTFEEDING
Although the literature has observed the absence of side effects on fetuses accidentally exposed to estrogen, the absence of a correct evaluation of the safety profile of these active ingredients does not allow their free use during pregnancy and breastfeeding.
Interactions
The activity of p450 cytochromial enzymes, responsible for the metabolism of ethinylestradiol, is subject to modulation by inhibitory and inducing active ingredients, such as antibiotics, phenobarbital and anticonvulsants.
The aforementioned variations may be responsible for significant pharmacokinetic variations such as to alter the therapeutic properties of ethinylestradiol, varying its efficacy and risk of side effects.
Contraindications ETHINYL ESTRADIOL Amsa ® - Ethinylestradiol
ETINYLESTRADIOL Amsa ® is contraindicated in case of untreated endometrial hyperplasia, venous thromboembolism, liver disease, porphyria, estrogen-dependent tumors, vaginal bleeding, breast cancer and related predisposing conditions, pregnancy and lactation and hypersensitivity to the active substance or to one of its excipients. .
Undesirable Effects - Side Effects
Hormone replacement therapy with estrogen exposes the patient to numerous potential side effects with both acute and chronic course.
The intake for prolonged periods of time or at high doses could favor the onset of nausea, vomiting, diarrhea, cramping abdominal pain, diffuse edema, increased breast volume and tenderness, neuro-psychiatric disorders, endometrial bleeding spots and gynecomastia in the " man.
Osteoporosis, increased risk of breast and endometrial carcinomas, cardiovascular diseases represent the most clinically relevant chronic pathological manifestations.
The risk of developing endometrial cancer is significantly higher in the absence of a combined progestogen therapy.
Note
ETHINYLESTRADIOL Amsa ® can only be sold under medical prescription.
The information on ETHINYLESTRADIOL Amsa ® - Ethinylestradiol published on this page may be out of date or incomplete. For a correct use of this information, see the Disclaimer and useful information page.