Generality
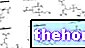
Threonine (T or THR) is an amino acid, which is one of the monomers that make up proteins; it is also a quaternary compound, since it is structured by four elements: carbon, hydrogen, oxygen and nitrogen. Its brute formula is C4H9NO3 and has a molecular weight of 119.12u (or Dalton).

Threonine is called a polar molecule; this means that in the side chain it contains charged groups at physiological pH, therefore they contribute to the so-called hydrogen bonds; the same side chain (with a hydroxyl OH) can be glycated, that is, it has the ability to bind to a glucose molecule. This characteristic is also shared by serine (another essential amino acid) and in the next paragraph we will understand why.
Furthermore, it is said that the structure of threonine (L-) is chiral, that is, that it cannot be superimposed on a mirror image (in the 3 dimensions); its enantiomer (hence specular) form is D-threonine.
Functions
From the metabolic point of view, in addition to constituting a plastic molecule of protein polymers (intended for the constitution of tissues, certain hormones, neurotransmitters, cell channels, immunoglobulins, etc.), threonine acts as a carrier (transporter) for the groups phosphate (PO43-) of phosphoproteins (eg milk caseins), thanks to the ability to receive them at the side chain. This process allows to transform the chemical-physical characteristics of the polymer in question and takes place by means of a protein-kinase catalyst enzyme. Of course, the best known phosphorylation is that of the transfer of the phosphate group from ATP (Adenosine Tri Phosphate) or GTP (Guanosin Tri Phosphate) to threonine or serine or tyrosine.
Nevertheless, threonine participates in many synthesis or metabolization reactions; for example, it is involved in the metabolism of creatine, other amino acids, cobalamin (vitamin B12), neurotransmitters (adrenaline and choline), etc.
How not to mention, then, its interaction with selenium, an antioxidant mineral, or the fundamental role in the hepatic metabolic processes of waste molecules.
ATTENTION! Based on a study by Young et al. (1989; Young & Pellet, 1990; Zello et al., 1995), like leucine, valine and lysine, threonine is also more subject to energy oxidation than the other amino acids, which is why it is logical to think that it may require a higher income in sports.
Food Sources and Shortages
Food sources of threonine are mainly: egg, dairy products, meat and fish.
The daily requirement of threonine is about 0.95g / day (8-20mg / kg in adults) and it is one of the amino acids that are most easily lacking in the vegan diet, that is the one totally devoid of ingredients of animal origin; for example, the limiting amino acid in rice and, after lysine, is also scarce in other widely consumed cereals.
Mild threonine deficiency can cause severe psychological irritability and personality disorders; the severe one is difficult to define but certainly very serious. The excess of threonine, on the other hand, is correlated to a "surge" of azotemia, with relative compromises of the organs responsible for metabolism and disposal of the excess in the long term.
Threonine supplements are marketed primarily for its potential to promote digestive functions, mental health, and the synthesis of collagen and elastin.