Active ingredients: Loratadine
Fristamin 10 mg tablets
Why is Fristamin used? What is it for?
The full name of this medicine is Fristamin tablets
What is Fristamin
Fristamin tablets contain the active substance loratadine which belongs to a class of medicines called 'antihistamines'.
How Fristamin works
Fristamin helps reduce allergic symptoms by blocking the effects of a substance called "histamine" which is produced by the human body when one is allergic to something.
When to take Fristamin
Fristamin relieves symptoms associated with allergic rhinitis (e.g. hay fever) such as: sneezing, runny and itchy nose, burning and itchy eyes in adults and children aged 2 years and over weighing more than 30 kg.
Fristamin is also used to relieve symptoms of hives (itching, redness and the number and size of hives).
The Fristamin effect lasts a full day and should help you continue your normal daily activities and sleep.
Contact your doctor if you do not notice any improvement or if you notice worsening of your symptoms.
Contraindications When Fristamin should not be used
Do not take Fristamin if:
you are allergic (hypersensitive) to loratadine or any of the other ingredients of this medicine (listed in section 6).
Precautions for use What you need to know before taking Fristamin
Talk to your doctor, pharmacist or nurse before taking Fristamin if:
- suffer from liver problems
- you must undergo skin tests for allergies. Do not take Fristamin in the two days before the tests, as this medicine may affect the results. If any of the above apply to you (or if you are not sure), talk to your doctor, pharmacist or nurse before taking Fristamin.
Children
Do not give Fristamin to children under the age of 2 or to children between the ages of 2 and 12 weighing less than 30 kg. For children aged
Interactions Which drugs or foods can modify the effect of Fristamin
The side effects of Fristamin may increase when taken together with medicines that alter the function of some enzymes responsible for the metabolism of the drug in the liver.
However, in clinical studies, there was no increase in the side effects of loratadine with products that alter the functioning of these enzymes.
Tell your doctor, pharmacist or nurse if you are taking, have recently taken or might take any other medicines, including medicines obtained without a prescription.
Fristamin and alcohol
Concomitant intake of Fristamin with alcoholic beverages has not been shown to potentiate its effects.
Warnings It is important to know that:
Pregnancy and breastfeeding
If you are pregnant, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
As a precautionary measure, it is preferable to avoid the use of Fristamin during pregnancy.
Do not take Fristamin if you are breastfeeding. Loratadine is excreted in breast milk.
Driving and using machines
In clinical studies performed to evaluate the ability to drive, no adverse effects were observed in patients treated with loratadine. At the recommended dose, Fristamin is not expected to cause you to be drowsy or less alert.
However, somnolence has occurred very rarely in some people, which may affect the ability to drive or use machines.
Fristamin contains lactose
Fristamin contains lactose; therefore, if you have been told by your doctor that you have "intolerance to some sugars, contact your doctor before taking this medicinal product.
Dose, Method and Time of Administration How to use Fristamin: Posology
Always take this medicine exactly as described in this leaflet or as directed by your doctor, pharmacist or nurse. If you are not sure, ask your doctor, pharmacist or nurse.
The score line is only there to help you break the tablet if you have difficulty swallowing it whole.
In what dose to take Fristamin:
Adults and children over 12 years of age:
Take one tablet once a day, with a glass of water, with or without food.
In children from 2 to 12 years of age, the dosage is based on weight:
- Body weight over 30 kg: Take one tablet once a day, with a glass of water, with or without food.
- Body weight less than or equal to 30 kg: Do not administer Fristamin. There are other formulations more suitable for children aged between 2 and 12 years and weighing less than or equal to 30 kg.
Fristamin is not recommended for children under 2 years of age.
Adults and children with severe liver problems:
- Adults and children weighing more than 30 kg: Take one tablet every other day, with a glass of water, with or without food.
Before taking this medicine, however, you should contact your doctor, pharmacist or nurse.
If you forget to take Fristamin
- If you forget to take your medicine, take it as soon as you remember, then carry on with your treatment as usual.
- Do not take a double dose to make up for a forgotten dose.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist or nurse.
Overdose What to do if you have taken too much Fristamin
If you take more Fristamin than you should, contact your doctor or pharmacist immediately.
No serious problems should arise, but headaches, rapid heartbeat, or sleepiness may occur.
Side Effects What are the side effects of Fristamin
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The most commonly reported side effects in adults and children over 12 years of age are:
- drowsiness
- headache
- increased appetite
- trouble sleeping.
The most commonly reported side effects in children aged 2 to 12 years are:
- headache
- nervousness
- tiredness
The following very rare side effects (may affect up to 1 in 10,000 people) have also been observed during the marketing of loratadine:
- severe allergic reaction (including swelling)
- dizziness
- convulsions
- fast or irregular heart rhythm
- nausea (feeling sick)
- dry mouth
- stomach upset
- liver trouble
- hair loss
- rash
- tiredness
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system. Agenziafarmaco.gov.it/it/responsabili. By reporting side effects you can help provide more information on the safety of this medicine.
Expiry and Retention
Keep this medicine out of the sight and reach of children.
This medicine does not require any special storage conditions.
Do not use this medicine after the expiry date which is stated on the blister after EXP. The expiry date refers to the last day of that month.
Do not use this medicine if you notice changes in the appearance of the tablet.
Do not throw any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. This will help protect the environment.
Deadline "> Other information
What Fristamin contains
- The active ingredient is loratadine. Each tablet contains 10 mg of loratadine.
- The other ingredients are lactose monohydrate, maize starch and magnesium stearate.
Description of what Fristamin looks like and contents of the pack
Tablet
White to off-white oval tablet, debossed with a flask and mortar, a score line and the number "10" on one side. Fristamin tablets are available in packs of 2, 5, 7, 10, 14, 15, 20, 21, 28, 30, 50, 60 or 100 tablets.
Not all pack sizes may be marketed
Source Package Leaflet: AIFA (Italian Medicines Agency). Content published in January 2016. The information present may not be up-to-date.
To have access to the most up-to-date version, it is advisable to access the AIFA (Italian Medicines Agency) website. Disclaimer and useful information.
01.0 NAME OF THE MEDICINAL PRODUCT -
FRISTAMIN 10 MG TABLETS
02.0 QUALITATIVE AND QUANTITATIVE COMPOSITION -
Each tablet contains 10 mg of loratadine.
Excipients with known effects: the amount of lactose monohydrate in the composition of the 10 mg loratadine tablet is 71.3 mg.
For the full list of excipients, see section 6.1.
03.0 PHARMACEUTICAL FORM -
Tablet
White to off-white oval tablet debossed with a flask and mortar on one side, a score line and the number "10" and plain on the other side.
The score line on the tablet is only to facilitate breaking for easier swallowing and not to divide into equal doses.
04.0 CLINICAL INFORMATION -
04.1 Therapeutic indications -
Fristamin is indicated for the symptomatic treatment of allergic rhinitis and chronic idiopathic urticaria in adults and children over 2 years of age with a body weight over 30 kg.
04.2 Posology and method of administration -
Dosage
Adults and children over 12 years of age: 10 mg once a day (one tablet once a day).
Pediatric population
In children from 2 to 12 years of age, the dosage is based on weight:
Body weight over 30 kg: 10 mg once a day (one tablet once a day).
Body weight less than or equal to 30 kg: the 10 mg tablet is not suitable for children weighing less than 30 kg. For children aged between 2 and 12 years and weighing less than or equal to 30 kg there are other more suitable formulations.
The safety and efficacy of Fristamin in children aged less than 2 years have not been established. There are no data available.
Patients with hepatic impairment
Patients with severe hepatic impairment should be given a lower starting dose as they may have reduced clearance of loratadine. A starting dose of 10 mg every other day is recommended in adults and children weighing more than 30 kg.
Patients with kidney damage
No dosage adjustments are required in patients with renal insufficiency.
Senior citizens
No dosage adjustments are required in the elderly.
Method of administration
Oral use. The tablet can be taken regardless of the time of meals.
04.3 Contraindications -
Hypersensitivity to the active substance or to any of the excipients listed in section 6.1.
04.4 Special warnings and appropriate precautions for use -
Fristamin should be administered with caution in patients with severe hepatic impairment (see section 4.2).
This medicine contains lactose; therefore patients with rare hereditary problems of galactose intolerance, the Lapp syndrome due to lactase deficiency or glucose-galactose malabsorption should not take this medicinal product.
Fristamin administration should be stopped at least 48 hours before skin tests as antihistamines can prevent or reduce positive reactions to skin reactivity indices.
04.5 Interactions with other medicinal products and other forms of interaction -
Concomitant intake of Fristamin with alcohol does not potentiate its effects, as assessed by psychophysical performance studies.
Potential interactions with all known inhibitors of CYP3A4 and CYP2D6 may occur resulting in elevated loratadine levels (see section 5.2), which may lead to an increase in adverse events.
Increased plasma concentrations of loratadine have been reported after concomitant use with ketoconazole, erythromycin and cimetidine in controlled clinical trials, but with no clinically significant changes (including electrocardiographic changes).
Pediatric population
Interaction studies have only been performed in adults.
04.6 Pregnancy and breastfeeding -
Pregnancy
Data on a large number of pregnant women exposed to the drug (over 1000 pregnancy outcomes) showed no malformative or fetal / neonatal toxicity effects of loratadine. Animal studies do not indicate direct or indirect harmful effects with respect to reproductive toxicity (see section 5.3). As a precaution it is preferable to avoid the use of Fristamin during pregnancy.
Feeding time
Loratadine is excreted in breast milk. Therefore, the use of Fristamin is not recommended in breastfeeding women.
Fertility
There are no data on fertility in men and women.
04.7 Effects on ability to drive and use machines -
In clinical studies performed to evaluate the ability to drive, no adverse effects were observed in patients treated with loratadine. Fristamin has no or negligible influence on the ability to drive or use machines. However, patients should be advised that somnolence has occurred very rarely and may affect their ability to drive or use machines.
04.8 Undesirable effects -
Summary of the safety profile
In clinical trials conducted in adult and adolescent subjects in a number of indications, including allergic rhinitis (RA) and chronic idiopathic urticaria (CIU), at the recommended dose of 10 mg per day, adverse reactions were reported with loratadine in a higher percentage. 2% compared to that of patients treated with placebo. The most common adverse reactions reported at a higher frequency than placebo were somnolence (1.2%), headache (0.6%), increased appetite (0.5%) and insomnia (0.1%).
Table of adverse reactions
The following adverse reactions, reported during the post-marketing period, are listed in the table below by system organ class. Frequencies are defined as very common (≥1 / 10), common (≥1 / 100,
Within each frequency category, adverse reactions are presented in order of decreasing severity.
Pediatric population
In clinical trials conducted in a pediatric population of children aged 2 to 12 years, the common adverse reactions reported in excess of placebo were headache (2.7%), nervousness (2.3%) and fatigue (1%).
Reporting of suspected adverse reactions
Reporting of suspected adverse reactions occurring after authorization of the medicinal product is important as it allows continuous monitoring of the benefit / risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system. "address http://www.agenziafarmaco.gov.it/it/responsabili.
04.9 Overdose -
Loratadine overdose increased the frequency of anticholinergic symptoms. Somnolence, tachycardia and headache have been reported following overdose.
In the event of an overdose, general symptomatic and supportive measures should be implemented and maintained for as long as necessary. Administration of activated charcoal suspended in water can be attempted. Gastric lavage may be considered. Loratadine is not eliminated by hemodialysis and it is not known whether it is eliminated by peritoneal dialysis. Medical monitoring of the patient should continue even after emergency treatment.
05.0 PHARMACOLOGICAL PROPERTIES -
05.1 "Pharmacodynamic properties -
Pharmacotherapeutic group: antihistamine - H1 antagonist, ATC code: R06A X13.
Mechanism of action
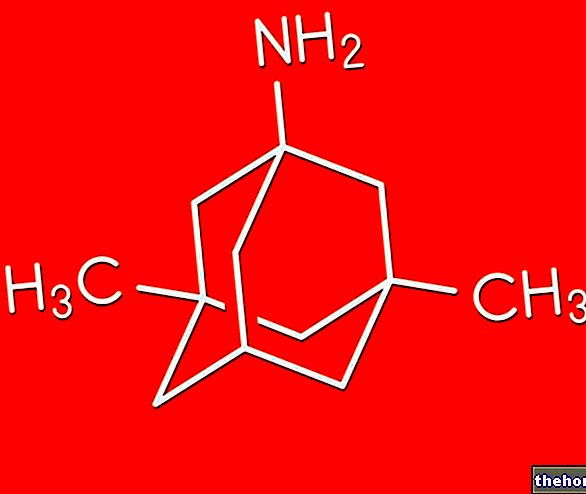
Loratadine, the active ingredient in Fristamin, is a tricyclic antihistamine with selective activity for peripheral H1 receptors.
Pharmacodynamic effects
Loratadine has no clinically significant sedative or anticholinergic properties in the majority of the population and when used at the recommended dosage.
There were no clinically significant changes in vital signs, laboratory parameters, physical examination, or electrocardiograms during long-term treatments.
Loratadine has no significant activity on H2 receptors. It does not inhibit the absorption of noradrenaline and practically does not affect cardiovascular function or the intrinsic activity of cardiac pacemakers.
Studies on the development of secondary histamine skin wheals in humans following administration of a single 10 mg dose have shown that antihistamine effects occur over 1 to 3 hours, peaking at 8 to 12 hours and lasting more than 24 hours. There was no evidence of tolerance to this effect after 28 days of loratadine administration.
Clinical efficacy and safety
Over 10,000 subjects (12 years of age and older) have been treated with loratadine 10 mg tablets in controlled clinical trials. Loratadine 10 mg tablets once daily was shown to be superior to placebo and similar to clemastine in improving the effects on nasal and non-nasal symptoms of allergic rhinitis. In these studies, a lower frequency of somnolence was observed with loratadine than with clemastine and approximately the same frequency as with terfenadine and placebo.
Among these subjects (12 years of age and older), 1,000 subjects with chronic idiopathic urticaria were enrolled in placebo-controlled studies. A 10 mg once daily dose of loratadine was superior to placebo in the management of chronic idiopathic urticaria, as evidenced by the reduction in associated pruritus, erythema and urticaria. In these studies, the incidence of somnolence with loratadine it was similar to that seen with placebo.
Pediatric population
Approximately 200 pediatric subjects (6 to 12 years of age) with seasonal allergic rhinitis received loratadine syrup in doses up to 10 mg once daily in controlled clinical trials. In another study, 60 pediatric subjects (aged 2 to 5 years) received loratadine syrup at a dose of 5 mg once daily. No unexpected adverse events were observed.
Pediatric efficacy was similar to efficacy observed in adults.
05.2 "Pharmacokinetic properties -
Absorption
Loratadine is well absorbed quickly. Concomitant ingestion of food may slightly delay the absorption of loratadine, but without influencing its clinical effect. The bioavailability parameters of loratadine and its active metabolite are dose proportional.
Distribution
Loratadine binds significantly to plasma proteins (97% to 99%) and its active metabolite - desloratadine (DL) - binds moderately (73% to 76%).
In healthy subjects, the plasma distribution half-lives of loratadine and that of its active metabolite are approximately 1 and 2 hours, respectively.
Biotransformation
After oral administration, loratadine is rapidly and well absorbed and undergoes significant first pass metabolism, mainly by CYP3A4 and CYP2D6. The major metabolite - desloratadine (DL) - is pharmacologically active and is responsible for much of the clinical effect. Loratadine and DL reach maximum plasma concentrations (Tmax) in 1 - 1.5 hours and 1.5 - 3.7, respectively. hours after administration.
Elimination
Approximately 40% of the administered dose is eliminated in the urine and 42% in the faeces, mainly in the form of conjugated metabolites, over a period of more than 10 days. Approximately 27% of the administered dose is excreted in the urine during the first 24 hours. Less than 1% of the active ingredient is excreted unchanged, in its active form, as loratadine or DL.
The mean elimination half-life in healthy adult subjects was 8.4 hours (range = 3 to 20 hours) for loratadine and 28 hours (range = 8.8 to 92 hours) for the major active metabolite.
Kidney damage
Both the AUC and maximum plasma levels (Cmax) of loratadine and its active metabolite were increased in patients with chronic renal disease compared to the same values in patients with normal renal function. The mean elimination half-life of loratadine and its active metabolite they did not differ significantly from those observed in normal subjects. Hemodialysis does not affect the pharmacokinetics of loratadine or its active metabolite in subjects with chronic renal failure.
Hepatic impairment
In patients with chronic alcohol-induced liver disease, the AUC and maximum plasma levels (Cmax) of loratadine were twice that of patients with normal hepatic function, while the pharmacokinetic profile of the active metabolite did not change significantly. The half-life. elimination of loratadine and that of its active metabolite were 24 hours and 37 hours, respectively, and increased with the severity of liver disease.
Senior citizens
The pharmacokinetic profile of loratadine and its active metabolite is comparable in healthy adult volunteers and healthy elderly volunteers.
05.3 Preclinical safety data -
Non-clinical data reveal no special hazard for humans based on conventional studies of safety, pharmacology, repeated dose toxicity, genotoxicity and carcinogenic potential.
In reproductive toxicity studies, no teratogenic effects were observed. However, prolonged calving times and reduced viability of the offspring were observed in rats at plasma concentrations (AUC) 10-fold higher than those achieved with clinical doses.
06.0 PHARMACEUTICAL INFORMATION -
06.1 Excipients -
Lactose monohydrate
Cornstarch
Magnesium stearate.
06.2 Incompatibility "-
Not relevant.
06.3 Period of validity "-
36 months
06.4 Special precautions for storage -
This medicine does not require any special storage conditions.
06.5 Nature of the immediate packaging and contents of the package -
Blisters consisting of 20 mcm aluminum foil with vinyl heat wrap and 250 mcm clear polyvinyl chloride film
Packs of 2, 5, 7, 10, 14, 15, 20, 21, 28, 30, 50, 60 or 100 tablets.
Not all pack sizes may be marketed.
06.6 Instructions for use and handling -
Unused medicine and wastes derived from this medicine must be disposed of in accordance with local regulations.
07.0 HOLDER OF THE "MARKETING AUTHORIZATION" -
SIGNATURE. S.p.A. - Via di Scandicci, 37 - Florence.
08.0 MARKETING AUTHORIZATION NUMBER -
Fristamin 10 mg tablets, 5 tablets AIC 027076052
Fristamin 10 mg tablets, 7 tablets AIC 027076064
Fristamin 10 mg tablets, 10 tablets AIC 027076076
Fristamin 10 mg tablets, 20 tablets AIC 027076013
09.0 DATE OF FIRST AUTHORIZATION OR RENEWAL OF THE AUTHORIZATION -
Date of first authorization:
Fristamin 10 mg tablets, 5 tablets 16/06/2003
Fristamin 10 mg tablets, 7 tablets 16/06/2003
Fristamin 10 mg tablets, 10 tablets 16/06/2003
Fristamin 10 mg tablets, 20 tablets 01/09/1989
Last renewal date: 08/11/2007
10.0 DATE OF REVISION OF THE TEXT -
March 2016