Active ingredients: Ethinylestradiol, Drospirenone
Yasminelle 0.02 mg / 3 mg film-coated tablets
Why is Yasminelle used? What is it for?
- Yasminelle is a contraceptive pill and is used to prevent pregnancy.
- Each tablet contains a small amount of two female hormones, drospirenone and ethinyl estradiol.
- Contraceptive pills that contain two hormones are called combination pills.
Contraindications When Yasminelle should not be used
When you shouldn't use Yasminelle
Do not use Yasminelle if you have any of the conditions listed below. If you have any of the conditions listed below, please contact your doctor. Your doctor will discuss with you other birth control methods that may be more suitable for you.
Do not take Yasminelle:
- if you have (or have ever had) a blood clot in a blood vessel of the leg (deep vein thrombosis, DVT), lung (pulmonary embolism, PE) or other organs;
- if you know you have a disorder that affects blood clotting, such as protein C deficiency, protein S deficiency, antithrombin-III deficiency, factor V Leiden or antiphospholipid antibodies;
- if you are going to have an "operation or if you will be lying down for a long time (see section" Blood clots "); if you have ever had a heart attack or stroke;
- if you have (or have ever had) angina pectoris (a condition that causes severe chest pain and may be a first sign of a heart attack) or transient ischemic attack (TIA - temporary stroke symptoms);
- if you have any of the following diseases, which could increase the risk of clots in the arteries: o severe diabetes with blood vessel damage o very high blood pressure o very high level of fat (cholesterol or triglycerides) in the blood o a disease known as hyperhomocysteinemia
- if you have (or have ever had) a type of migraine called 'migraine with aura';
- if you have (or have ever had) liver disease and your liver function is still abnormal
- if your kidneys are not working well (kidney failure)
- if you have (or have ever had) liver cancer
- if you have (or have ever had) or if you are suspected of having breast or genital cancer
- if you have unexplained vaginal bleeding
- if you are allergic to ethinyl estradiol or drospirenone or any of the other ingredients of this medicine (listed in section 6). This condition can cause itching, rash or swelling.
Precautions for use What you need to know before taking Yasminelle
Before you start using Yasminelle you should read the information on blood clots in section 2. It is especially important that you read the symptoms of a blood clot (see section 2 "Blood clots"). Before taking Yasminelle, your doctor will ask you a few questions about your personal health history and that of your family members. The doctor will also measure your blood pressure and, depending on your personal situation, may also carry out other tests. This leaflet describes various situations in which you must stop Yasminelle or in which the safety of Yasminelle may be decreased. In such situations it is necessary to refrain from sexual intercourse or to take additional non-hormonal contraceptive measures, such as a condom or other barrier method. Do not use the rhythm or basal temperature method. In fact, these methods can be unreliable as Yasminelle alters the monthly changes in body temperature and cervical mucus. Yasminelle, like all hormonal contraceptives, offers no protection against HIV infection (AIDS) or other sexually transmitted diseases.
Interactions Which drugs or foods can change the effect of Yasminelle
Always tell your doctor if you are taking any medicines or herbal products. Tell any other doctor or dentist who prescribes another medicine (or pharmacist) that you are taking Yasminelle. They will be able to tell you if you need to take additional contraceptive measures (e.g. condoms) and for how long.
Some medicines affect the blood levels of Yasminelle and can make it less effective in preventing pregnancy or can cause unexpected bleeding. These include:
- medicines to treat: epilepsy (e.g. primidone, phenytoin, barbiturates, carbamazepine, oxcarbazepine) or tuberculosis (e.g. rifampicin) or infections with HIV and Hepatitis C virus (ritonavir, nevirapine, efavirenz known as protease inhibitors and inhibitors non-nucleoside reverse transcriptase) or other infections (griseofulvin) or blood pressure at the lungs (bosentan)
- St. John's wort herbal remedy. Yasminelle may affect the effect of other medicines, for example: medicines containing cyclosporine;
- the anti-epileptic lamotrigine (this can lead to an increase in the frequency of seizures). Ask your doctor or pharmacist for advice before taking any medicine.
Yasminelle with food and drink
Yasminelle can be taken with or without food, with a little water if needed.
Laboratory analysis
If you need to have a blood test, tell your doctor or laboratory staff that you are taking the pill, as hormonal contraceptives can affect the results of some tests.
Pregnancy
If you are pregnant, you should not use Yasminelle. If you become pregnant while taking Yasminelle you must stop immediately and contact your doctor. If you wish to become pregnant, you can stop taking Yasminelle at any time (see also "If you stop taking Yasminelle")
Ask your doctor or pharmacist for advice before taking any medicine.
Feeding time
The use of Yasminelle is generally not recommended during breastfeeding. If you want to take the pill while breastfeeding, you should contact your doctor.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
There is no evidence that Yasminelle affects the ability to drive or use machines.
Yasminelle contains lactose
If you cannot tolerate some sugars, contact your doctor before taking Yasminelle.
Warnings It is important to know that:
When should you see a doctor?
Contact a doctor urgently
- if you notice possible signs of a blood clot which may indicate that you are suffering from a blood clot in the leg (deep vein thrombosis), a blood clot in the lung (pulmonary embolism), a heart attack or a stroke (see section below " Blood clots ").
For a description of the symptoms of these serious side effects go to the section "How to recognize a blood clot".
Tell your doctor if any of the following apply to you.
In some situations you need to be extra careful while using Yasminelle or any other combination pill and your doctor may need to see you regularly. If this condition appears or worsens while you are using Yasminelle, you should tell your doctor.
- if a close relative has or has ever had breast cancer
- if you have liver or gallbladder disease
- if you have diabetes
- if you suffer from depression
- if you have Crohn's disease or ulcerative colitis (chronic inflammatory bowel disease);
- if you have systemic lupus erythematosus (SLE, a disease that affects the natural defense system);
- if you have haemolytic uremic syndrome (HUS, a blood clotting disorder causing kidney failure);
- if you have sickle cell anemia (an inherited disease of the red blood cells);
- if you have high levels of fat in the blood (hypertriglyceridaemia) or a "positive family history of this condition." Hypertriglyceridaemia has been associated with an increased risk of developing pancreatitis (inflammation of the pancreas);
- if you are going to have an "operation or if you are going to lie down for a long time (see section 2" Blood clots ");
- if you have just given birth, your risk of developing blood clots is higher. Ask your doctor how long after having a baby you can start taking Yasminelle;
- if you have "inflammation of the veins under the skin (superficial thrombophlebitis);
- if you have varicose veins.
- if you have epilepsy (see "Other medicines and Yasminelle")
- if you have a disease that first appeared during pregnancy or previous use of sex steroids (for example, hearing loss, a blood disorder called porphyria, rash with blisters during pregnancy (herpes gravidarum) ), a nerve disease causing sudden body movements (Sydenham's chorea))
- if you have or have ever had chloasma (discoloration of the skin, especially of the face or neck, known as "pregnancy spots"). In this case, avoid direct exposure to sunlight or ultraviolet rays
- if you have hereditary angioedema, medicines containing estrogen can cause or worsen the symptoms. If you experience symptoms of angioedema, such as swelling of the face, tongue and / or throat and / or difficulty in swallowing or hives with difficulty in breathing, contact your doctor immediately.
BLOOD CLOTS
Using a combined hormonal contraceptive such as Yasminelle increases your risk of developing a blood clot compared with not using one. In rare cases, a blood clot can block blood vessels and cause serious problems.
Blood clots can develop
- in veins (called "venous thrombosis", "venous thromboembolism" or VTE)
- in the arteries (referred to as 'arterial thrombosis', 'arterial thromboembolism' or ATE).
Recovery from blood clots is not always complete. Rarely, long-lasting severe effects can occur or, very rarely, they can be fatal.
It is important to remember that the overall risk of a harmful blood clot associated with Yasminelle is low.
HOW TO RECOGNIZE A BLOOD CLOT
See a doctor immediately if you notice any of the following signs or symptoms.
- swelling of one leg or along a vein in the leg or foot, especially when accompanied by:
- pain or tenderness in the leg which may only be felt when standing or walking
- increased sensation of heat in the affected leg
- change in color of the skin on the leg, such as turning pale, red or blue
- sudden and unexplained shortness of breath or rapid breathing;
- sudden cough with no obvious cause, possibly causing blood to be emitted;
- sharp chest pain which may increase with deep breathing;
- severe light headedness or dizziness;
- rapid or irregular heartbeat;
- severe stomach pain If you are unsure, tell your doctor as some of these symptoms such as coughing or shortness of breath may be mistaken for a milder condition such as a "respiratory infection (eg a" common cold " ).
- immediate loss of vision or
- painless blurring of vision which can progress to loss of vision
- chest pain, discomfort, feeling of pressure or heaviness
- sensation of squeezing or fullness in the chest, arm or below the breastbone;
- feeling of fullness, indigestion or choking;
- upper body discomfort radiating to the back, jaw, throat, arms and stomach;
- sweating, nausea, vomiting or dizziness;
- extreme weakness, anxiety, or shortness of breath;
- rapid or irregular heartbeats
- sudden numbness or weakness of the face, arm or leg, especially on one side of the body;
- sudden confusion, difficulty speaking or understanding;
- sudden difficulty seeing in one or both eyes;
- sudden difficulty walking, dizziness, loss of balance or coordination;
- sudden, severe or prolonged migraine with no known cause;
- loss of consciousness or fainting with or without seizures. Stroke symptoms can sometimes be brief, with almost immediate and complete recovery, but you still need to see a doctor urgently as you may be at risk for another stroke.
- swelling and pale blue discoloration of one "extremity;
- severe stomach pain (acute abdomen)
BLOOD CLOTS IN A VEIN
What can happen if a blood clot forms in a vein?
- The use of combined hormonal contraceptives has been linked to an increased risk of blood clots forming in the veins (venous thrombosis). However, these side effects are rare. In most cases they occur in the first year of using a combined hormonal contraceptive.
- If a blood clot forms in a vein in the leg or foot, it can cause a deep vein thrombosis (DVT).
- If a blood clot travels from the leg and lodges in the lung, it can cause a "pulmonary embolism."
- Very rarely, a clot can form in another organ such as the eye (retinal vein thrombosis).
When is the risk of developing a blood clot in a vein highest?
The risk of developing a blood clot in a vein is highest during the first year of taking a combined hormonal contraceptive for the first time. The risk may be even higher if you restart taking a combined hormonal contraceptive (the same drug or a different drug) after a break of 4 or more weeks.
After the first year, the risk is reduced but is always slightly higher than if you were not using a combined hormonal contraceptive.
When you stop taking Yasminelle, the risk of developing a blood clot returns to normal within a few weeks.
What is the risk of developing a blood clot?
The risk depends on your natural risk of VTE and the type of combined hormonal contraceptive you are taking.
The overall risk of developing a blood clot in the leg or lung (DVT or PE) with Yasminelle is low.
- Out of 10,000 women who are not using any combined hormonal contraceptive and who are not pregnant, about 2 will develop a blood clot in a year.
- Out of 10,000 women who are using a combined hormonal contraceptive that contains levonorgestrel, norethisterone or norgestimate, about 5-7 will develop a blood clot in a year.
- Out of 10,000 women who are using a combined hormonal contraceptive containing drospirenone, such as Yasminelle, about 9-12 will develop a blood clot in a year.
- The risk of a blood clot forming depends on your medical history (see under "Factors that increase the risk of a blood clot forming").
Factors that increase the risk of developing a blood clot in a vein
The risk of developing a blood clot with Yasminelle is low but some conditions increase the risk. Its risk is greater:
- if you are severely overweight (body mass index or BMI over 30 kg / m2);
- if a close relative has had a blood clot in the leg, lung or other organ at a young age (less than about 50 years). In this case you could have an inherited blood clotting disorder;
- if you are going to have an operation or if you have to lie down for a long time because of an injury or illness or if you have a leg in a cast. You may need to stop taking Yasminelle a few weeks before the surgery or in the period in which you are less mobile. If you have to stop taking Yasminelle, ask your doctor when you can start taking it again;
- as you get older (especially over the age of 35);
- if you gave birth less than a few weeks ago. The risk of developing a blood clot increases the more conditions you have of this type.
Air travel (lasting> 4 hours) may temporarily increase the risk of a blood clot, especially if you have some of the other risk factors listed.
It is important that you tell your doctor if any of these apply to you, even if you are not sure. Your doctor may decide that Yasminelle needs to be stopped.
If any of the above conditions change while you are using Yasminelle, for example if a close relative has a thrombosis for no known reason or if you gain a lot of weight, contact your doctor.
BLOOD CLOTS IN AN ARTERY
What can happen if a blood clot forms in an "artery?"
Like blood clots in a vein, clots in an artery can cause serious problems, for example, they can cause a heart attack or stroke.
Factors that increase the risk of developing a blood clot in an artery
It is important to note that the risk of heart attack or stroke associated with the use of Yasminelle is very low but can increase:
- with increasing age (over 35 years);
- if you smoke. When using a combined hormonal contraceptive such as Yasminelle you are advised to stop smoking. If you are unable to stop smoking and are over the age of 35, your doctor may advise you to use a different type of contraceptive;
- if you are overweight;
- if you have high blood pressure;
- if a member of your immediate family has had a heart attack or stroke at a young age (less than about 50 years). In this case, you may also be at high risk of having a heart attack or stroke;
- if you or a close relative have a high level of fat in the blood (cholesterol or triglycerides);
- if you suffer from migraines, especially migraines with aura;
- if you have any heart problems (valve defect, a heart rhythm disorder called atrial fibrillation);
- if you have diabetes.
If you have more than one of these conditions or if any of them are particularly severe, the risk of developing a blood clot may be even higher.
If any of the above conditions change while you are using Yasminelle, for example if you start smoking, if a close relative has a thrombosis for no known reason, or if you gain a lot of weight, contact your doctor.
Yasminelle and cancer
Breast cancer is seen slightly more frequently in women using combination pills, but it is not known whether this is due to the treatment. For example, it is possible that more cancers are diagnosed in women who use the Pill because they undergo more frequent medical checks. The occurrence of breast cancer gradually decreases after stopping the combined pill. It is important that you check your breasts regularly and contact your doctor if you feel any lump.
Benign liver tumors and, even more rarely, malignant liver tumors have been observed in rare cases in women using the Pill. Contact your doctor if you experience unusually severe abdominal pain.
Intermenstrual bleeding
During the first few months of taking Yasminelle you may experience unexpected bleeding (bleeding outside the one week off). If this bleeding occurs for more than a few months, or starts after a few months, your doctor should check what is wrong.
What to do if menstruation does not appear during the week off
If you have taken all the tablets correctly, have not had vomiting or severe diarrhea and have not taken any other medicines, it is highly unlikely that you are pregnant.
If the period does not appear twice in a row, she may be pregnant. Contact your doctor immediately. Do not start the next strip until you are sure you are not pregnant.
Dose, Method and Time of Administration How to use Yasminelle: Posology
Take one Yasminelle tablet every day, with a little water if needed. You can take the tablets with or without food, but you must take them around the same time each day.
The blister contains 21 tablets. Next to each tablet is printed the day of the week it should be taken. For example, if you start on a Wednesday, take the tablet next to WED. Follow the direction of the arrows on the blister until you have taken all 21 tablets.
So do not take tablets for 7 days. During these 7 days (so-called withdrawal week) menstruation should appear. This is called "withdrawal bleeding" and usually starts on the second or third day of the withdrawal week.
Start a new strip on the eighth day after the last Yasminelle tablet (ie after the 7 day break), regardless of whether or not your period has stopped. This means that you must start each new Yasminelle blister on the same day of the week and your period will also start on the same day each month.
By taking Yasminelle as indicated above, you are protected from pregnancy even during the 7 days that you do not take the tablets.
When can the first blister start?
- If you have not used a hormonal contraceptive in the previous month
Start taking Yasminelle on the first day of your period (i.e. the first day of your period). If you start on the first day of your period, the contraceptive effect is immediate. You can also start taking Yasminelle between the 2nd and 5th day of your period, but in this case you must take additional contraceptive measures (for example a condom) to the first 7 days.
- Changing from a combined hormonal contraceptive or a combined contraceptive vaginal ring or patch
Start taking Yasminelle preferably the day after the last active tablet (the last tablet containing the active ingredients) of the previous pill, or at the latest the day after the end of the pill-free interval (or after the last inactive tablet. of the previous pill). When switching from a combined contraceptive vaginal ring or patch, follow your doctor's advice.
- Changing from a progestogen-only method (progestogen-only pill, injection, implant, or progestogen-releasing intrauterine system (IUS))
You can switch any day from the progestogen-only pill (from an implant or IUS on the day of its removal, from an injectable when you have the next injection), but in all these cases take additional contraceptive measures (eg. condom) for the first 7 days of taking the tablets.
- After an abortion
Follow your doctor's advice.
- After a birth
You can start taking Yasminelle between day 21 and day 28 after giving birth. If you start later than day 28, use a so-called barrier method (eg condoms) during the first 7 days of taking Yasminelle . If, after having a baby, you have had intercourse before starting Yasminelle (or restarting), make sure you are not pregnant or wait for your period.
- If you are breastfeeding and want to start (or restart) Yasminelle
Read the "Breastfeeding" section.
Ask your doctor for advice if you are not sure when to start.
Overdose What to do if you have taken too much Yasminelle
If you take more Yasminelle than you should
There are no reports of serious harmful effects of taking too many Yasminelle tablets.
If you take several tablets at once, you may feel sick or vomit. Young girls can have vaginal bleeding.
If you have taken too many Yasminelle tablets, or if you find that a child has taken some tablets, contact your doctor or pharmacist immediately.
If you forget to take Yasminelle
- If you are less than 12 hours late in taking a tablet, contraceptive protection is not reduced. Take the tablet as soon as you remember and then take the next tablets as planned.
- If you are more than 12 hours late in taking a tablet, contraceptive protection may be reduced. The more tablets you miss, the greater the risk of becoming pregnant.
The risk of incomplete contraceptive protection is greatest if you forget a tablet at the beginning or at the end of the strip. You should therefore follow the instructions below (see also the diagram below):
- More than one tablet forgotten in this pack
Talk to your doctor.
- One tablet forgotten in the first week
Take the forgotten tablet as soon as you remember, even if that means that you have to take two tablets at the same time. Then continue taking the tablets at the usual time and take additional contraceptive measures for the next 7 days, for example a condom. If you have had sexual intercourse in the week preceding the forgetfulness, you may be pregnant. In this case, please contact your doctor.
- One tablet forgotten in the second week
Take the forgotten tablet as soon as you remember, even if that means that you have to take two tablets at the same time. Then continue taking the tablets at the usual time. The contraceptive safety of the pill is maintained and it is therefore not necessary to take additional precautions.
- One tablet forgotten in week 3
You can choose between two possibilities:
- you can take the forgotten tablet as soon as you remember, even if that means taking two tablets at the same time. Continue taking the tablets at the usual time. Skip the tablet-free interval and start the next strip immediately. You will most likely not have a period until the second strip is finished, but you may also have low or menstrual bleeding during the second strip.
- you can also stop taking the tablets from your current cycle and go directly to the 7-day break (including the day of the forgotten tablet). If you want to start the next strip on your usual day, keep an interval of less than 7 days. If you follow either of these two recommendations, you will stay protected against pregnancy.
- If you have forgotten any of the tablets in the strip and do not menstruate during the first tablet-free interval, you may be pregnant.Talk to your doctor before starting a new strip.

What to do in case of vomiting or severe diarrhea
If you vomit within 3-4 hours after taking a tablet or have severe diarrhea, the active substances in the pill may not be fully absorbed by your body. The situation is comparable to when you forget to take a tablet. After vomiting or diarrhea, take another tablet from a reserve pack as soon as possible. If possible, take it within 12 hours of the usual tablet-taking time. If this is not possible, or if 12 hours have already passed, you should follow the instructions in the section 'If you forget to take Yasminelle'.
How to delay menstruation: what you need to know
Although not recommended, you can delay your period by continuing with a new Yasminelle strip instead of observing the 7-day interval. You may experience low or menstrual bleeding while using this second strip. Continue with the next strip after the usual 7-day interval.
You can ask your doctor for advice before deciding to delay your period.
How to change the start day of your period: what you need to know
If you take the tablets according to the instructions, your period will start in the week off. If you have to change the start day, shorten the normal break between two packs (but never extend it - 7 days is the maximum!). For example, if your break normally starts on a Friday and you want to move it to Tuesday (3 days earlier), you start the next blister pack 3 days early. If you make the break between two cycles very short (eg 3 days or less), you may not have bleeding during this interval.
Later, you may have low or menstrual bleeding.
If you are not sure what to do, ask your doctor for advice
If you stop taking Yasminelle
You can stop taking Yasminelle at any time. If you still want to avoid becoming pregnant, ask your doctor for advice on other safe birth control methods. If you want to become pregnant, stop taking Yasminelle and wait for your period before trying to get pregnant.
This will allow you to more easily calculate the expected date of delivery.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
Side Effects What are the side effects of Yasminelle
Like all medicines, this medicine can cause side effects, although not everybody gets them. If you get any side effects, especially if they are severe or persistent, or if there is any change in your health that you think might be due to Yasminelle, please tell your doctor.
An increased risk of developing blood clots in the veins (venous thromboembolism (VTE)) or blood clots in the arteries (arterial thromboembolism (ATE)) is present in all women taking combined hormonal contraceptives. For more detailed information on the different risks from "taking combined hormonal contraceptives, see section 2" What you need to know before you use Yasminelle ".
The following side effects have been associated with the use of Yasminelle.
Common side effects (may affect between 1 and 10 in 100 women):
- mood swings
- headache
- abdominal pain (stomach pain)
- acne
- breast pain, breast enlargement, breast tenderness, irregular or painful periods
- weight gain.
Uncommon side effects (may affect between 1 and 10 in 1000 women):
- Candidiasis (fungal infection)
- cold sores (herpes simplex)
- allergic reactions
- increased appetite
- depression, nervousness, sleep disturbances
- pins and needles, dizziness
- poor eyesight
- irregular or unusually fast heartbeat
- blood clot (thrombosis) in the lungs (pulmonary embolism), high blood pressure, low blood pressure, migraine, varicose veins
- sore throat
- nausea, vomiting, inflammation of the stomach and / or intestines, diarrhea, constipation
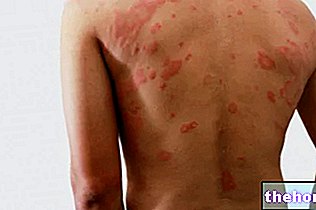
- sudden swelling of the skin and / or mucous membranes (e.g. tongue or throat) and / or difficulty in swallowing or hives with difficulty in breathing (angioedema), hair loss (alopecia), eczema, itching, rash, dryness skin, oily skin (seborrheic dermatitis)
- neck pain, pain in limbs, muscle cramps
- bladder infection
- breast lump (benign and cancer), milk production outside pregnancy (galactorrhea), ovarian cysts, hot flashes, no menstruation, very heavy periods, vaginal discharge, vaginal dryness, pain in the lower abdomen (pelvic), abnormal vaginal smear (PAP test), decreased interest in sex
- water retention, lack of energy, excessive thirst, increased sweating
- weight loss.
Rare side effects (may affect between 1 and 10 in 10,000 women):
- asthma
- damage to hearing
- erythema nodosum (characterized by painful, reddish skin nodules)
- erythema multiforme (rash with target lesions or ulcers).
- harmful blood clots in a vein or artery, for example: in a leg or foot (DVT) in a lung (PE) heart attack stroke mini-stroke or temporary stroke-like symptoms, known as a stroke transient ischemic blood clots in the liver, stomach / intestines, kidneys or eye.
The chance of developing a blood clot may be higher if you have any other conditions that increase this risk (see section 2 for more information on conditions that increase the risk of blood clots and the symptoms of a blood clot).
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system at https://www.aifa.gov.it/content/segnalazioni-reazioni-avverse. By reporting side effects you can help provide more information on safety. of this medicine.
Expiry and Retention
Keep this medicine out of the sight and reach of children.
This medicine does not require any special storage conditions.
Do not use this medicine after the expiry date which is stated on the package after "Do not use after" or "EXP."
Do not throw any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. This will help protect the environment.
Composition and pharmaceutical form
What Yasminelle contains
- The active ingredients are ethinyl estradiol (as betadxtrin clathrate) and drospirenone. Each tablet contains 0.020 milligrams of ethinyl estradiol (as betadxtrin clathrate) and 3 milligrams of drospirenone.
- The other ingredients are lactose monohydrate, maize starch, magnesium stearate (E470b), hypromellose (E464), talc (E553b), titanium dioxide (E171), red iron oxide (E172).
What Yasminelle looks like and contents of the pack
- Each blister of Yasminelle contains 21 light pink film-coated tablets.
- Yasminelle tablets are film-coated; the tablet core is coated. The tablets are light pink, round, with convex faces, one of which is embossed with the letters "DS" in a regular hexagon.
- Yasminelle is available in packs of 1, 3, 6 and 13 blisters, each with 21 film-coated tablets.
Not all pack sizes may be marketed.
Source Package Leaflet: AIFA (Italian Medicines Agency). Content published in January 2016. The information present may not be up-to-date.
To have access to the most up-to-date version, it is advisable to access the AIFA (Italian Medicines Agency) website. Disclaimer and useful information.
01.0 NAME OF THE MEDICINAL PRODUCT
YASMINELLE 0.02 MG / 3 MG TABLETS COATED WITH FILM
02.0 QUALITATIVE AND QUANTITATIVE COMPOSITION
Each tablet contains 0.020 mg of ethinylestradiol (as betdextrin clathrate) and 3 mg of drospirenone.
Excipient with known effect: 46 mg lactose
For the full list of excipients, see section 6.1.
03.0 PHARMACEUTICAL FORM
Film-coated tablets.
Round, light pink tablets with convex faces, one of which is embossed with the letters "DS" in a regular hexagon.
04.0 CLINICAL INFORMATION
04.1 Therapeutic indications
Oral contraception.
The decision to prescribe Yasminelle must take into account the individual woman's current risk factors, particularly those related to venous thromboembolism (VTE) and the comparison between the risk of VTE associated with Yasminelle and that associated with other combined hormonal contraceptives (COCs). (see sections 4.3 and 4.4).
04.2 Posology and method of administration
Method of administration: oral use.
Dosage
How to take Yasminelle
The tablets should be taken at about the same time each day, if needed with a small amount of liquid, and in the order indicated on the blister pack. The dosage is one tablet per day for 21 consecutive days. Each subsequent pack should be started afterwards. an interval of seven days, during which a "withdrawal bleed" usually occurs. This usually starts 2-3 days after taking the last tablet and may not have finished before the next pack is started.
How to start treatment with Yasminelle
• No previous use of hormonal contraceptives (in the previous month)
The first tablet should be taken on the first day of your natural menstrual cycle (i.e. the first day of your period).
• Changing from a combined hormonal contraceptive (combined oral contraceptive, vaginal ring or transdermal patch)
Yasminelle should preferably be started on the day after the last active tablet (the last tablet containing the active ingredients) of the previous COC, or at the latest on the day after the usual tablet-free interval or after the last one. placebo tablet of the previous combined oral contraceptive. If a vaginal ring or transdermal patch has been used, Yasminelle should preferably be started on the day of removal, or at the latest when the next application should be made.
• Changing from a progestogen-only contraceptive (progestogen-only pill, injection, implant) or from a progestogen-releasing intrauterine system (IUS)
The woman can switch to Yasminelle at any time if she is using the progestogen-only pill (in the case of an implant or IUS, the day of its removal; in the case of an injectable, the day it should be given. " injection); however, in all these cases, the woman should be advised to use an additional barrier method of contraception for the first 7 days of dosing.
• After an abortion in the first trimester of pregnancy
You can start immediately, without the need for extra contraceptive measures.
• After a birth or abortion in the second trimester of pregnancy
Tablet-taking should begin between the 21st and 28th day after delivery or abortion in the second trimester of pregnancy. In case of later initiation, the woman should be advised to use an additional barrier contraceptive method for the first few months. 7 days However, if you have had sexual intercourse in the meantime, you must either rule out pregnancy, or wait until your next period, before you start using the COC.
For breastfeeding women see section 4.6.
Behavior in case of failure to take tablets
If she is less than 12 hours late in taking a tablet, contraceptive protection is maintained. The woman should take the tablet as soon as she remembers and then take the following tablets at the usual time.
If you are more than 12 hours late in taking a tablet, contraceptive protection may be reduced. If you miss a tablet, the following principles apply:
1. Tablet-taking must never be discontinued for more than 7 days.
2. It takes 7 days of uninterrupted tablet-taking to achieve "adequate suppression of the hypothalamus-pituitary-ovarian axis."
As a result, the following advice can be given in daily practice:
• 1st week
The forgotten tablet should be taken as soon as the woman remembers, even if this involves taking two tablets at the same time. Then she should continue to take the tablets regularly as planned. In addition, contraception is required for the next 7 days. such as a condom. If intercourse occurred in the previous 7 days, the possibility of a pregnancy should be considered. The greater the number of missed tablets, the closer to the free interval. from the pill, the higher the risk of pregnancy.
• 2nd week
The forgotten tablet should be taken as soon as the woman remembers, even if this involves taking two tablets at the same time. Then she should continue to take the tablets regularly as planned. If the tablets have been taken correctly within 7 days use of additional contraceptive methods is not necessary. However, if more than one tablet has been missed, the use of additional precautions should be recommended for 7 days.
• 3rd week
Given the imminent 7-day tablet-free interval, the risk of reduced contraceptive reliability is greater. However, by changing the tablet-taking schedule, the reduction in contraceptive protection can still be prevented. If one of the following two options is used, therefore, it is not necessary to use additional contraceptive measures, provided that in the 7 days preceding the first missed tablet all tablets have been taken correctly. If not, it is necessary to follow the first of the two options and also to take additional contraceptive measures in the following 7 days.
1. The forgotten tablet should be taken as soon as the woman remembers, even if this involves taking two tablets at the same time. Then she should continue to take the tablets regularly as planned. The next pack should be started immediately after the end. than the one in use, ie without observing any pause between the two packs. Withdrawal bleeding is unlikely until the second pack is finished, however, spotting or breakthrough bleeding may occur while taking the tablets.
2. You may also be advised to stop taking the tablets from the current pack. In this case, you should observe a tablet-free interval of up to 7 days, including those in which the tablets have been missed, and then resume. with a new packaging.
If the woman has forgotten to take tablets and does not experience withdrawal bleeding in the first regular tablet-free interval, the possibility of an ongoing pregnancy should be considered.
Recommendations in case of gastrointestinal disorders
In case of severe gastrointestinal disturbances (eg vomiting or diarrhea), absorption may be incomplete and additional contraceptive measures must be used.
If vomiting occurs within 3-4 hours of taking a tablet, a new (replacement) tablet should be taken as soon as possible. If possible, the new tablet should be taken within 12 hours of the usual taking time. 12 hours, the same instructions for forgetting tablets apply as described in section 4.2 "Behavior in case of missing tablets".
If you do not want to change the usual dosing schedule, you will have to take the tablet (s) you need from another pack.
How to move a "withdrawal bleed."
To delay a period, you should continue with another pack of Yasminelle without observing the pill-free interval.This can be continued for as long as desired until the end of the second pack. During this prolonged use, breakthrough bleeding or spotting may occur. Yasminelle should be resumed regularly after the usual 7-day tablet-free interval.
To shift your period to another day of the week than it occurs with your current schedule, you may be advised to shorten your first pill-free interval by the desired days. The shorter this interval, the greater the chance of not having a withdrawal bleed and you will experience breakthrough bleeding or spotting during the next pack (such as when you want to delay your period).
04.3 Contraindications
Combined hormonal contraceptives (COCs) should not be used in the following conditions. Should any of these conditions occur for the first time during COC use, the medicinal product should be discontinued immediately.
• Presence or risk of venous thromboembolism (VTE)
• Venous thromboembolism - current (with anticoagulant intake) or previous VTE (eg deep vein thrombosis [DVT] or pulmonary embolism [PE])
• Known hereditary or acquired predisposition to venous thromboembolism, such as resistance to activated protein C (including factor V Leiden), antithrombin III deficiency, protein C deficiency, protein S deficiency
• Major surgery with prolonged immobilization (see section 4.4)
• High risk of venous thromboembolism due to the presence of multiple risk factors (see section 4.4)
• Presence or risk of arterial thromboembolism (ATE)
• Arterial thromboembolism - current or previous arterial thromboembolism (eg myocardial infarction) or prodromal conditions (eg angina pectoris)
• Cerebrovascular disease - current or previous stroke or prodromal conditions (eg transient ischemic attack (transient ischaemic attack, TIA))
• Known hereditary or acquired predisposition to arterial thromboembolism, such as hyperhomocysteinaemia and antiphospholipid antibodies (anticardiolipin antibodies, lupus anticoagulant)
• History of migraine with focal neurological symptoms
• A high risk of arterial thromboembolism due to the presence of multiple risk factors (see section 4.4) or the presence of a serious risk factor such as:
• diabetes mellitus with vascular symptoms
• severe hypertension
• severe dyslipoproteinemia
• severe hepatic disease, current or previous, until the values of liver function return to normal;
• severe or acute renal failure;
• existing or previous liver tumors (benign or malignant);
• Known or suspected malignant diseases, dependent on sex steroids (eg of the genital organs or the breast);
• vaginal bleeding of an unknown nature;
• hypersensitivity to the active substances or to any of the excipients listed in section 6.1.
04.4 Special warnings and appropriate precautions for use
Warnings
• If any of the conditions or risk factors mentioned below are present, the suitability of Yasminelle should be discussed with the woman.
• If any of these risk factors or conditions worsen or first appear, the woman should contact her doctor to determine if the use of Yasminelle should be discontinued.
• In the case of suspected or confirmed VTE or ATE, the use of COCs should be discontinued. If anticoagulant therapy is started, an alternative method of contraception should be used because of the teratogenic risk associated with anticoagulant therapy (coumarins).
• Circulatory Disorders
Risk of venous thromboembolism (VTE)
The use of any combined hormonal contraceptive (COC) results in an increased risk of venous thromboembolism (VTE) compared with no use. Products that contain levonorgestrel, norgestimate or norethisterone are associated with a lower risk of VTE. The risk associated with others. products such as Yasminelle can also be twofold. The decision to use a product other than those associated with a lower risk of VTE should only be made after discussions with the woman to ensure that she understands the risk of VTE associated with Yasminelle, the way where your current risk factors influence that risk and the fact that the risk of developing a VTE is highest in the first year of use. There is also some evidence that the risk increases when taking a COC is resumed after a break of 4 or more weeks.
About 2 in 10,000 women who do not use a CHC and who are not pregnant will develop a VTE over a period of one year. In a single woman, however, the risk can be much higher, depending on her underlying risk factors (see below).
It is estimated that out of 10,000 women who use a CHC containing drospirenone, between 9 and 12 will develop a VTE in one year; this compares with about 6 women using a COC containing levonorgestrel.
In both cases, the number of VTEs per year is less than the number expected in pregnancy or in the postpartum period.
VTE can be fatal in 1-2% of cases.
Very rarely, thrombosis has been reported in CHC users in other blood vessels, e.g. hepatic, mesenteric, renal or retinal veins and arteries.
Risk factors for VTE
The risk of venous thromboembolic complications in CHC users may increase substantially if additional risk factors are present, especially if there are more than one risk factors (see table).
Yasminelle is contraindicated if a woman has several risk factors that increase her risk of venous thrombosis (see section 4.3). If a woman has more than one risk factor, it is possible that the increased risk is greater than the sum of the individual factors; in this case her total risk of VTE should be considered. If the benefit-risk ratio is considered to be negative, a COC should not be prescribed (see section 4.3).
Table: Risk factors for VTE
There is no agreement on the possible role of varicose veins and superficial thrombophlebitis in the onset and progression of venous thrombosis.
The increased risk of thromboembolism in pregnancy, particularly the 6-week period of the puerperium, must be considered (for information on "Pregnancy and lactation" see section 4.6).
Symptoms of VTE (deep vein thrombosis and pulmonary embolism)
If symptoms of this type occur, women should seek immediate medical attention and inform them that they are taking a CHC.
Symptoms of deep vein thrombosis (DVT) can include:
- unilateral swelling of the leg and / or foot or along a vein in the leg;
- pain or tenderness in the leg which may only be felt when standing or walking;
- increased sensation of heat in the affected leg; skin on the leg that is red or discolored.
Symptoms of pulmonary embolism (PE) can include:
- sudden and unexplained onset of shortness of breath and rapid breathing;
- sudden cough which may be associated with hemoptysis;
- sharp pain in the chest;
- severe light headedness or dizziness;
- rapid or irregular heartbeat.
Some of these symptoms (such as "shortness of breath" and "cough") are non-specific and may be misinterpreted as more common or less serious events (eg respiratory tract infections).
Other signs of vascular occlusion may include: sudden pain, swelling or a pale blue discoloration of one "extremity.
If the occlusion takes place in the eye, symptoms can range from painless blurring of vision to loss of vision. Sometimes vision loss occurs almost immediately.
Risk of arterial thromboembolism (ATE)
Epidemiological studies have associated the use of CHCs with an increased risk of arterial thromboembolism (myocardial infarction) or cerebrovascular accidents (eg transient ischemic attack, stroke). Arterial thromboembolic events can be fatal.
Risk factors of ATE
The risk of arterial thromboembolic complications or a cerebrovascular accident in CHC users increases in the presence of risk factors (see table). Yasminelle is contraindicated if a woman has one serious risk factor or multiple risk factors for ATE that increase her risk of arterial thrombosis (see section 4.3). If a woman has more than one risk factor, it is possible that the increase in risk is greater than the sum of the individual factors; in this case her total risk should be considered. If the benefit-risk balance is believed to be negative, a CHC should not be prescribed (see section 4.3).
Table: Risk factors of ATE
Symptoms of ATE
If symptoms of this type occur, women must contact a healthcare professional immediately and inform them that they are taking a CHC.
Symptoms of a cerebrovascular accident can include:
• sudden numbness or weakness of the face, arm or leg, especially on one side of the body;
• sudden difficulty walking, dizziness, loss of balance or coordination;
• sudden confusion, difficulty speaking or understanding;
• sudden difficulty seeing in one or both eyes;
• sudden, severe or prolonged migraine with no known cause;
• loss of consciousness or fainting with or without convulsions.
Temporary symptoms suggest it is a transient ischemic attack (TIA).
Symptoms of myocardial infarction (MI) can include:
• pain, discomfort, pressure, heaviness, sensation of squeezing or fullness in the chest, arm or below the breastbone;
• discomfort radiating to the back, jaw, throat, arms, stomach;
• feeling of fullness, indigestion or choking;
• sweating, nausea, vomiting or dizziness;
• extreme weakness, anxiety or shortness of breath;
• rapid or irregular heartbeats.
• Tumors
An increased risk of cervical cancer in COC users for long periods (> 5 years) has been reported in some epidemiological studies, but it is still controversial to what extent this finding is attributable to confounding effects of sexual and other behavior. factors such as human papilloma virus (HPV).
A meta-analysis of 54 epidemiological studies found that women currently using COCs have a slightly increased relative risk (RR = 1.24) of having breast cancer diagnosed. The excess risk gradually disappears over the 10 years following discontinuation of COCs. Because breast cancer is rare in women under the age of 40, the extra number of breast cancers diagnosed in women using or who have recently used COCs is small in relation to the overall risk of breast cancer. . Such studies provide no evidence of a causal relationship. The observed increased risk may be due to an earlier diagnosis of breast cancer in COC users, the biological effects of COCs, or a combination of both. Breast cancer diagnosed in COC users combined tends to be less clinically advanced than that diagnosed in women who have never used them.
Benign liver tumors and, even more rarely, malignant liver tumors have been reported rarely in women taking COCs. In isolated cases, these tumors have resulted in life-threatening intra-abdominal haemorrhage. If a woman taking a combined oral contraceptive develops severe upper abdominal pain, liver enlargement, or signs suggestive of intra-abdominal haemorrhage, the possibility of liver cancer should be considered in the differential diagnosis.
With the use of higher-dosed COCs (50 mcg ethinylestradiol) the risk of endometrial and ovarian cancer is reduced. Whether this also applies to lower-dosed COCs remains to be confirmed.
• Other conditions
The progestogen component of Yasminelle is an aldosterone antagonist with potassium sparing properties. In most cases, no increases in potassium levels are to be expected. In a clinical study, however, in some patients with mild renal impairment or moderate concomitant taking potassium-sparing medicinal products, serum potassium levels increased slightly, but not significantly, while taking drospirenone. Therefore, it is recommended to monitor serum potassium during the first course of treatment in patients with renal insufficiency and who have a pre-treatment serum potassium value in the upper part of the reference range, particularly if they are taking concomitant medications. potassium sparing See also section 4.5.
Women with hypertriglyceridaemia or a family history of the condition may have an increased risk of pancreatitis when using COCs.
Although a small increase in blood pressure has been reported in many women taking COCs, a clinically relevant increase is rare. Only in these rare cases is an immediate discontinuation of COCs justified. If, during the use of a COC in a patient with pre-existing hypertension, blood pressure is constantly elevated or a significant increase in blood pressure does not respond adequately to antihypertensive therapy, the combined oral contraceptive should be discontinued. If deemed appropriate, COC use may be resumed if blood pressure has normalized following antihypertensive therapy.
The onset or worsening of the following conditions has been reported both during pregnancy and while taking COCs: jaundice and / or itching due to cholestasis, gallstone formation, porphyria, systemic lupus erythematosus, haemolytic uremic syndrome, chorea Sydenham's, herpes gravidarum, otosclerosis hearing loss; however, there is no conclusive evidence of a correlation between these conditions and the use of COCs.
In women with hereditary angioedema, exogenous estrogens can induce or aggravate the symptoms of angioedema.
Acute or chronic disturbances of liver function may require discontinuation of COC treatment until liver function indices have returned to normal.The recurrence of cholestatic jaundice and / or cholestatic pruritus already occurring in pregnancy or during previous sex steroid treatment requires discontinuation of the COC.
Although COCs may have an effect on peripheral insulin resistance and glucose tolerance, there is no evidence for the need to change the treatment regimen in diabetic patients using low-dose combined oral contraceptives (containing
Worsening of endogenous depression, epilepsy, Crohn's disease and ulcerative colitis has been reported during COC use.
Chloasma may occasionally occur, especially in women with a history of chloasma gravidarum. Women with a tendency to chloasma should avoid exposure to the sun or ultraviolet rays while using COCs.
This medicinal product contains 46 mg of lactose per tablet. Patients with rare hereditary problems of galactose intolerance, lactase deficiency or glucose-galactose malabsorption who are on a lactose-free diet should take this rate into account.
Medical examination / visits
Before initiating or resuming use of Yasminelle, a complete medical history (including family history) should be taken and pregnancy excluded. Blood pressure should be measured and a clinical examination, guided by contraindications, should be performed (see section 4.3) and warnings (see section 4.4). It is important to draw a woman's attention to information relating to venous or arterial thrombosis, including the risk associated with Yasminelle compared to other CHCs, symptoms of VTE and ATE, known risk and what to do in case of suspected thrombosis.
The woman should also be advised of the need to read the package leaflet carefully and follow its advice. The frequency and type of examinations should be based on established guidelines and should be adapted to the individual woman.
Women should be advised that hormonal contraceptives do not protect against HIV infection (AIDS) or other sexually transmitted diseases.
Reduced effectiveness
The efficacy of COCs may decrease in case of missed tablet intake (see section 4.2), gastrointestinal disturbances (see section 4.2) or concomitant administration of other medicinal products (see section 4.5).
Reduced cycle control
Irregular vaginal bleeding (spotting or breakthrough bleeding) may occur with all COCs, especially in the first months of use. Therefore, evaluation of any irregular bleeding is meaningful only after a settling period of approximately three treatment cycles.
If irregular bleeding persists or occurs after previously regular cycles, a non-hormonal etiology should be considered and adequate diagnostic measures should be implemented to rule out malignancy or pregnancy. Such measures may include scraping.
In some women, withdrawal bleeding may not occur during the tablet-free days. If the COC has been taken according to the directions given in section 4.2, it is unlikely that the woman is pregnant. However, if the COC has not been taken according to directions prior to the first missed withdrawal bleed, or if two withdrawal bleeds have not occurred, pregnancy must be ruled out before COC use is continued.
04.5 Interactions with other medicinal products and other forms of interaction
Note: The information in the Summary of Product Characteristics of concomitant medicinal products should be consulted to identify possible interactions.
• Effects of other medicines on Yasminelle
Interactions with drugs that induce microsomal enzymes can occur resulting in increased clearance of sex hormones and can cause breakthrough bleeding and / or contraceptive failure.
Management
Enzyme induction can already be observed after a few days of treatment. Maximal enzyme induction is generally observed within a few weeks. After discontinuation of therapy, enzyme induction may persist for approximately 4 weeks.
Short-term treatment
Women undergoing enzyme inducer treatments should temporarily use a barrier method or another method of contraception in addition to the combined oral contraceptive. The barrier method should be used for the entire period of concomitant drug intake and for 28 days following discontinuation of therapy. If therapy continues after the end of the active tablets of the COC pack, the placebo tablets should be discarded and the next COC pack started.
Long-term treatment
For women undergoing long-term treatment with hepatic enzyme inducers, another reliable, non-hormonal method of contraception is recommended.
The following interactions have been reported in the literature.
Substances increasing the clearance of COCs (decreased efficacy of COCs by enzyme inducers)
Barbiturates, bosentan, carbamazepine, phenytoin, primidone, rifampicin, the HIV drug ritonavir, nevirapine and efavirenz and possibly also felbamate, griseofulvin, oxycarbazepine, topiramate and products containing "St. John's wort" (Hypericum perforatum).
Substances with variable effect on the clearance of COCs
When co-administered with COCs, combinations of HIV protease inhibitors and non-nucleoside reverse transcriptase inhibitors, including combinations with HCV inhibitors, may increase or decrease plasma concentrations of estrogen or progestogen. The net effect of these changes in some cases may be clinically relevant.
Consequently, prescribing information regarding HIV / HCV concomitant medications should be consulted to identify potential interactions and any related recommendations. If in doubt, the woman undergoing therapy with protease inhibitors or non-nucleoside reverse transcriptase inhibitors should use a barrier method of contraception.
The major metabolites of drospirenone in human plasma are produced without the involvement of the cytochrome P450 system. Inhibitors of this enzyme system are unlikely to affect the metabolism of drospirenone.
• Effects of Yasminelle on other medicines
Oral contraceptives can affect the metabolism of some active ingredients. Consequently, plasma and tissue concentrations of these may increase (e.g. cyclosporine) or decrease (e.g. lamotrigine).
Based on inhibition studies in vitro and interaction studies in vivo performed in female volunteers using omeprazole, simvastatin and midazolam as marker substrates, an interaction of drospirenone at a dose of 3 mg with the metabolism of other active substances is unlikely.
• Other forms of interaction
In patients without renal insufficiency, concomitant use of drospirenone and ACE inhibitors or NSAIDs has not been shown to exert a significant effect on serum potassium. However, concomitant use of Yasminelle with aldosterone antagonists or potassium-sparing diuretics did not In this case, serum potassium should be monitored during the first treatment cycle See also section 4.4.
• Laboratory tests
The use of contraceptive steroids can affect the results of some laboratory tests, including biochemical parameters relating to liver, thyroid, adrenal and renal function, plasma levels of (carrier) proteins, such as binding globulin. corticosteroids and lipid / lipoprotein fractions, parameters of glucose metabolism and parameters of coagulation and fibrinolysis. Changes generally remain within normal limits. Drospirenone causes an increase in plasma renin activity and plasma aldosterone, a due to its weak antimineralocorticoid activity.
04.6 Pregnancy and breastfeeding
Pregnancy
Yasminelle is not indicated during pregnancy.
In the event of pregnancy occurring while using Yasminelle, the medicinal product should be discontinued immediately. Large epidemiological studies have revealed neither increased risk of congenital malformations in children born to women who have used COCs before. of pregnancy, nor teratogenic effects in case of accidental intake of COCs during pregnancy.
Animal studies have shown undesirable effects during pregnancy and lactation (see section 5.3). Based on these animal data, undesirable effects due to the hormonal action of the active substances cannot be excluded. However, general clinical experience with COCs during pregnancy did not provide any evidence of a real adverse effect in man.
The available data on the use of Yasminelle in pregnancy are too limited to draw any conclusions on the adverse effects of Yasminelle on pregnancy or on the health of the fetus or newborn. To date no relevant epidemiological data are available.
The increased risk of thromboembolism in the postpartum period should be taken into account when Yasminelle is restarted (see sections 4.2. And 4.4).
Pregnancy
Breastfeeding can be affected by COCs, as they can decrease the quantity and change the composition of breast milk. Therefore, the use of COCs should not normally be recommended until weaning is done. been completed. Small amounts of contraceptive steroids and / or their metabolites may be excreted in breast milk during COC use. Such amounts may affect the baby.
04.7 Effects on ability to drive and use machines
No studies on the ability to drive and use machines have been performed. No effect on the ability to drive or use machines has been observed in COC users.
04.8 Undesirable effects
For serious undesirable effects in COC users see also section 4.4.
The following adverse reactions have been reported during the use of Yasminelle:
The table below shows adverse reactions by system organ according to MedDRA (MedDRA SOC). Frequencies are derived from clinical trial data.
The most appropriate MedDRA term was used to describe a specific reaction, its synonyms and related conditions.
Description of some adverse reactions
An increased risk of arterial and venous thrombotic and thromboembolic events, including myocardial infarction, stroke, transient ischemic attacks, venous thrombosis and pulmonary embolism has been observed in CHC users, and this risk is discussed in more detail in section 4.4.
The following serious adverse reactions, discussed in section 4.4, have been observed in women using COCs "Special warnings and precautions for use":
• venous thromboembolic disorders;
• arterial thromboembolic disorders;
• hypertension;
• liver tumors;
• onset or worsening of conditions for which the association with the use of COCs has not been definitively demonstrated: Crohn's disease, ulcerative colitis, epilepsy, uterine myoma, porphyria, systemic lupus erythematosus, herpes gravidarum, Sydenham's chorea , hemolytic-uremic syndrome, cholestatic jaundice;
• chloasma;
• Chronic or acute disturbances of liver function may require discontinuation of COCs until liver function indices have returned to normal;
• in women with hereditary angioedema, exogenous estrogens can induce or aggravate the symptoms of angioedema.
The frequency of breast cancer diagnoses among COC users increased very slightly. Because breast cancer is rare in women under the age of 40, the extra number of cases is small compared to the overall risk of breast cancer. It is not known whether there is a causal link with COCs. For further information see sections 4.3 and 4.4.
Interactions
Interaction between oral contraceptives and other drugs (enzyme inducers) may cause breakthrough bleeding and / or contraception failure (see section 4.5).
Reporting of suspected adverse reactions
Reporting of suspected adverse reactions occurring after authorization of the medicinal product is important as it allows continuous monitoring of the benefit / risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system. "address https://www.aifa.gov.it/content/segnalazioni-reazioni-avverse.
04.9 Overdose
There is currently no experience of overdose with Yasminelle. Based on general experience with COCs, symptoms that may occur in this case are: nausea, vomiting and, in young girls, mild vaginal bleeding. There is no antidote and treatment should be symptomatic.
05.0 PHARMACOLOGICAL PROPERTIES
05.1 Pharmacodynamic properties
Pharmacotherapeutic group (ATC): progestogens and estrogens, fixed combinations, ATC code G03AA12.
Pearl Index for method failure: 0.11 (upper bound of the bilateral 95% confidence interval: 0.60).
Overall Pearl Index (method failure + patient error): 0.31 (upper limit of the bilateral 95% confidence interval: 0.91).
The contraceptive effect of Yasminelle is based on the interaction of various factors, the most important of which are the inhibition of ovulation and the changes occurring in the endometrium.
Yasminelle is a combined oral contraceptive containing ethinylestradiol and the progestin drospirenone. At the therapeutic dose, drospirenone also possesses antiandrogenic properties and weak antimineralocorticoid properties. It is devoid of estrogenic, glucocorticoid and antiglucocorticoid activity. This gives drospirenone a similar pharmacological profile to that of natural progesterone.
Data from clinical studies indicate that the weak antimineralocorticoid properties of Yasminelle translate into weak antimineralocorticoid activity.
05.2 Pharmacokinetic properties
• Drospirenone
Absorption
After oral administration drospirenone is rapidly and almost completely absorbed. Maximum concentrations of the active ingredient of about 38 ng / ml are reached 1-2 hours after a single intake. Bioavailability is between 76 and 85%. Simultaneous ingestion of food has no influence on the bioavailability of drospirenone.
Distribution
After oral administration, serum drospirenone levels decrease with a terminal half-life of 31 hours. Drospirenone binds to serum albumin, but not to sex hormone-binding globulin (SHBG) or corticoid-binding globulin (CBG). Only 3-5% of the total concentrations of the active substance in serum are present in the form of free steroid. The ethinylestradiol-induced increase in SHBG does not affect the serum protein binding of drospirenone. The mean apparent volume of distribution of drospirenone is 3.7 ± 1.2 L / kg.
Biotransformation
After oral administration drospirenone is completely metabolised. The major metabolites in plasma are the acid form of drospirenone, produced by the opening of the lactone ring, and 4,5-dihydro-drospirenone-3-sulfate, both produced without involvement of the P450 system. Drospirenone is metabolised to a lesser extent by cytochrome P450 3A4 and has been shown to inhibit in vitro this enzyme and the cytochromes P450 1A1, P450 2C9 and P450 2C19.
Elimination
The metabolic clearance of drospirenone in serum is 1.5 ± 0.2 ml / min / kg. Drospirenone is eliminated in unchanged form only in trace amounts. The metabolites of drospirenone are excreted in the faeces and urine in a ratio of approximately 1.2 - 1.4. The half-life of metabolite excretion with urine and faeces is approximately 40 hours.
Steady-state conditions
During a course of treatment, steady-state maximum serum concentrations of drospirenone of approximately 70 ng / ml are reached after approximately 8 days of treatment. An accumulation of drospirenone serum levels by a factor of approximately 3 occurs as a consequence of the relationship between the terminal half-life and the interval between doses.
Special patient populations
Effect of impaired renal function
Steady-state serum drospirenone levels in women with mild renal impairment (creatinine clearance CLcr, 50-80 ml / min) are comparable to those in women with normal renal function. Serum levels of drospirenone are on average 37% higher in women with moderate renal impairment (CLcr, 30-50 mL / min) than in women with normal renal function. Drospirenone treatment is also well tolerated by women with mildly and moderately impaired renal function. Treatment with drospirenone shows no clinically significant effect on serum potassium concentration.
Effect of impaired liver function
In a single dose study in volunteers with moderate hepatic impairment, oral clearance (CL / F) was decreased by approximately 50% compared to that of patients with normal hepatic function. The reduction in clearance observed in volunteers with moderate hepatic impairment did not result in clear differences in serum potassium concentrations. Even in the presence of diabetes and concomitant treatment with spironolactone (two factors that can predispose to hyperkalaemia), no increase in serum potassium above the upper limit of normal has been observed. It can be concluded that drospirenone is well tolerated in patients with mild or moderate hepatic impairment (Child-Pugh B).
Ethnic groups
No relevant differences in the pharmacokinetics of drospirenone or ethinylestradiol were observed between Japanese and Caucasian women.
• Ethinylestradiol
Absorption
After oral administration, ethinylestradiol is rapidly and completely absorbed. Maximum plasma concentrations of approximately 33 pg / ml are reached within 1-2 hours after single intake. Absolute bioavailability is approximately 60%, as a consequence of presystemic conjugation and of first pass metabolism. The simultaneous ingestion of food reduced the bioavailability of ethinylestradiol in about 25% of the subjects studied, while no change was observed in the others.
Distribution
The serum levels of ethinylestradiol decrease with a biphasic trend and the terminal phase of elimination is characterized by a "half-life of about 24 hours. The" ethinylestradiol is largely bound to "serum albumin (about 98.5%), but in a way nonspecific, and induces an increase in serum concentrations of SHBG and corticoid-binding globulin (CBG) An apparent volume of distribution of approximately 5 L / kg has been calculated.
Biotransformation
Ethinylestradiol is subject to presystemic conjugation both in the mucosa of the small intestine and in the liver. Ethinylestradiol is metabolised mainly by aromatic hydroxylation, but a large variety of hydroxylated and methylated metabolites are formed, which are present both as free metabolites and as conjugates with glucuronides and sulphates. The metabolic clearance of ethinylestradiol is approximately 5 ml / min / kg.
Elimination
Ethinylestradiol is not eliminated to a significant extent in unchanged form. The metabolites of ethinylestradiol are eliminated at a urine / bile ratio of 4: 6. The half-life of metabolite excretion is approximately 1 day.
Steady-state conditions
Steady-state conditions are achieved during the second half of a treatment cycle and serum ethinylestradiol levels show an accumulation of a factor of approximately 2.0 - 2.3.
05.3 Preclinical safety data
In laboratory animals the effects of drospirenone and ethinylestradiol are limited to those associated with their recognized pharmacological activity. In particular, reproductive toxicity studies have revealed embryotoxic and foetotoxic effects in animals which are considered species-specific. At exposures above those occurring in users of Yasminelle, effects on sexual differentiation were observed in rat fetuses, but not in monkeys.
06.0 PHARMACEUTICAL INFORMATION
06.1 Excipients
Core of the tablet
Lactose monohydrate,
Cornstarch,
Magnesium stearate (E470b)
Tablet coating
hypromellose (E464),
talc (E553b),
titanium dioxide (E171),
red iron oxide (E172).
06.2 Incompatibility
Not relevant.
06.3 Period of validity
5 years.
06.4 Special precautions for storage
This medicine does not require any special storage conditions.
06.5 Nature of the immediate packaging and contents of the package
Transparent PVC / aluminum blister.
Packs of:
21 tablets.
3x21 tablets.
6x21 tablets.
13x21 tablets.
Not all pack sizes may be marketed.
06.6 Instructions for use and handling
Unused medicine and waste derived from this medicine must be disposed of in accordance with local regulations.
07.0 MARKETING AUTHORIZATION HOLDER
Bayer S.p.A. - Viale Certosa, 130 - 20156 Milan (MI)
08.0 MARKETING AUTHORIZATION NUMBER
1x21 film-coated tablets AIC n. 037199015
3x21 film-coated tablets AIC n. 037199027
6x21 film-coated tablets AIC n. 037199039
13x21 film-coated tablets AIC n. 037199041
09.0 DATE OF FIRST AUTHORIZATION OR RENEWAL OF THE AUTHORIZATION
23 November 2006/04 August 2010
10.0 DATE OF REVISION OF THE TEXT
04/2015