At the University of Pennsylvania, in fact, a group of researchers, following a study carried out on mice, identified a group of cells that would be responsible for the breakdown of the bone typical of the disease.
This discovery, if also confirmed by subsequent studies carried out on humans, could mark a turning point in the treatment of osteoporosis. The hypothesis is that by acting on the cells identified through this study on mice, in the future new therapies may be put in place for block or slow down their action and, consequently, the phenomenon of bone loss.
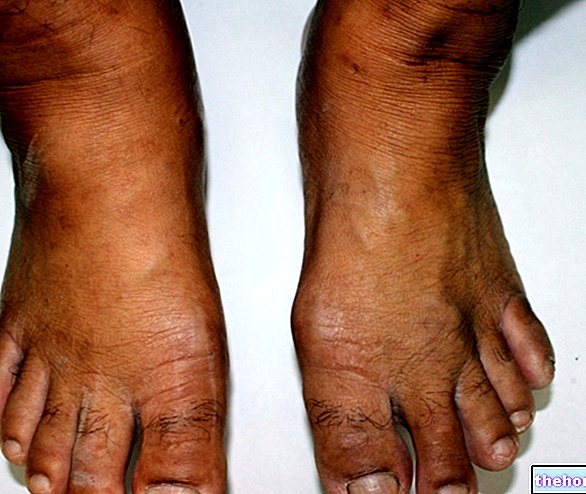
, increases the risk of trauma and fractures, especially of the femur, wrist, humerus, vertebrae and ankle.Women are most affected
There are two main types of osteoporosis: one called primitive, which is the most widespread and affects postmenopausal women or the elderly in general, and a secondary one, which can affect subjects of any age suffering from chronic diseases or under treatment with drugs that directly or indirectly adversely affect skeletal health.
As for the first type, it is estimated that in Italy it affects one in 3 women over 50 (about 5,000,000 people) and one out of 8 men over 60 (about 1,000,000 people).
How it develops
Normally and at any stage of life, the bone undergoes a physiological remodeling process, during which the old and damaged tissue is removed by the osteoclasts and the new one reformed by the osteoblasts.
As the years pass, the activity of the osteoclasts increases compared to that of the osteoblasts and this generates a natural loss of bone mass.
When this loss is further acute and bone resorption becomes significantly greater than its formation, osteoporosis appears.
to change over time, play a fundamental role.The results showed that it would be some defective mechanisms inside this process that determine the bone disintegration which then, in turn, cause the onset of osteoporosis.
Knowledge before the new discovery
Prior to this revolutionary discovery, scientists were already aware that the balance between osteoclasts and osteoblasts represented the aspect around which the entire process of maintaining a healthy bone revolves.
In the face of this basic knowledge, the aspect that until recently had remained obscure to researchers was what determined the variation of the osteoclasts and their becoming hyperactive and disrupting the bone before it could even reform, and what was the role coated with malp cells in this process.
Trying to understand this, the working group, in March 2020 had taken the first step, demonstrating how these precursors were able to produce the Rankl protein, considered essential for the formation of osteoclasts.
The phases of the research
Starting from those results, a more in-depth study was then started on rodents with Rankl deficiencies in their Malp cells.
At the end it emerged that the rodents, reached the month of life, had higher density in the spongy components of the long bones such as the femur, in an amount ranging from 60 to 100%. A significant discovery given that this is a very important increase compared to what is normally the bone mass of a mouse.
In the next step, the researchers identified in malp and their secretion of the Rankl protein, the triggering factors that regulate the bone absorption function performed by osteoclasts.
.
"If Rankl's secretion could be deactivated, this could help rebalance the bone remodeling process in those suffering from osteoporosis, allowing osteoblasts to catch up with osteoclasts", continues Link Qin, effectively paving the way for possible advancements. in the therapeutic field.
There is still a long way to go because experimentation must be transferred from mice to humans and its validity confirmed there, but if this were the case, the therapeutic scenario in the field of osteoporosis could really change.
If the results are established, in fact, the hope of the researchers is that it will be possible to exploit some advanced techniques such as genetic editing to regulate and control the behavior of cells, in order to block the process of bone loss.