Initially, the prospectus for waiting times for the first vaccines was more than a year.
However, some pharmaceutical and biotechnology companies involved in the trial have forged ahead and managed to distribute the first vaccine preparations approved already between the end of December 2020 and January 2021.
As of April 2021, there are four anti-COVD-19 vaccines approved by the FDA and EMA and in distribution, and several are still undergoing clinical trials or awaiting approval.
This article aims to analyze the types of COVID-19 vaccine available and those for which research is still ongoing, describing in a simple way the mechanism of operation of each.
Clearly, before getting to the heart of the discussion, it is good to review broadly what a vaccine is and what the principle of operation of vaccinations is.
For further information: New Coronavirus Vaccine: Research and Approval or oral, which allow to create an "immunization against one or more specific infectious pathogens (viruses, bacteria, etc.).
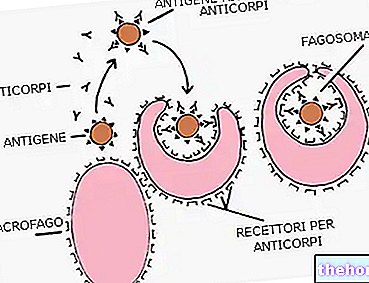
Vaccines are designed to stimulate the immune system and produce the antibodies needed for immunization.
To be successful, vaccines must contain something that belongs to the pathogen against which you want to get immunized; better known as antigen, this something can be:
- The pathogen itself in an attenuated (attenuated vaccines) or dead (inactivated vaccines) form;
- The inactive form of a protein, toxin or polysaccharide of the microorganism (subunit or purified antigen vaccines);
- A trait of genetic material of the pathogen, obviously bioengineered to be harmless (mRNA vaccines and viral vector vaccines).
The antigen present in vaccines is such as to trigger immune activity, but not to cause disease.
Vaccines have no immediate effect; the antibody response, in fact, takes from 2 to 4 weeks to create the desired and necessary immunization to defend against pathogens.