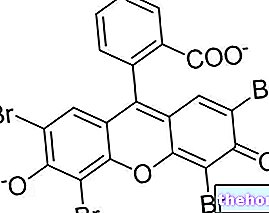
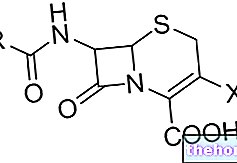
In organic chemistry, with the term raceme - or racemic mixture - s "means a 1: 1 (hence equimolar) mixture of two enantiomers. Enantiomers are defined as mirror images of the same molecule that cannot be superimposed on each other.
Organic molecules that cannot be superimposed on their own mirror image are called molecules chiral; exactly as our left hand is not superimposable on our right hand (from the Greek cheir "hand", from which the term chiral was born).

Example of the two enantiomers of a raceme
An organic molecule is chiral if - within its structure - it has a tetrahedral atom (usually a carbon atom but can also be a different atom) bonded to four different atoms or groups. A carbon atom that binds four atoms or groups different from each other and devoid of elements of symmetry is said chiral center or center of chirality.
To better understand the concept of chirality, the following is an example of the enantiomers of 2-butanol:
As you can see, the two molecules are one the mirror image of the other. They have the same atoms - linked in the same way - but oriented in a different way in space and this makes them non-superimposable.
Enantiomers are distinguished from each other based on the absolute configuration of the chiral center. The system still used today to assign the absolute configuration to the chiral centers is defined Cahn-Ingold-Prelog convention or convention R, S, named after the scientists who conceived it in the late 1950s.
Furthermore, enantiomers can be distinguished on the basis of their optical rotational power. In fact, molecules having chiral centers in their structure possess the ability to rotate the plane of polarized light; they are said to be composed optically active. However, there are also chiral molecules that are unable to rotate polarized light.
If a molecule rotates the plane of polarized light clockwise - then from left to right - it is called right-handed or dextrorotatory. If, on the other hand, the molecule rotates the light counterclockwise - therefore from right to left - it is defined left-handed or levorotatory.
A right-handed molecule is usually indicated by prefixing its name with the sign "+"or the letter"d", while a left-handed molecule is indicated by prefixing its name with the sign"-"or the letter"L'.
For each pair of enantiomers, one is dextrorotatory and the other is left-handed, but the absolute value of the optical rotational power is the same. Therefore, a raceme - in which there is an equimolar mixture of enantiomers and therefore contains the same number of dextrorotating and levorotatory molecules - possesses no optical rotational power, and is defined as optically inactive.