Characteristics and use as a sweetener
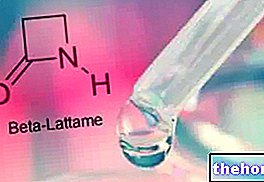
Aspartame is an artificial dipeptide composed of two common amino acids: aspartic acid and phenylalanine (whose carboxyl end is esterified with methanol).
Accidentally discovered in 1965 by chemist James Schlatter, of G. D. Searle and Company, aspartame has enjoyed extraordinary commercial success; this sweetener was in fact approved in the 1980s as a food sweetener and as such used on a large scale in soft drinks containing carbonic acid, powdered soft drinks, yoghurt and confectionery products and dietetics.

The taste of aspartame is described as "clean and sweet", devoid of the bitter or metallic aftertaste often associated with other synthetic sweeteners. Comparison with sucrose reveals that the taste is similar to that of natural sugars; furthermore, some flavors are present. in foods and drinks they are enhanced or prolonged in the presence of aspartame, especially those of acidic fruits (such as orange and lemon). This property is exploited in chewing gum, where the aromas can be prolonged for 4 times longer.
The sweetening power of aspartame is 160-220 times greater than sucrose, while the caloric intake is more or less equivalent (4 Kcal / gram, like any protein). Consequently, very few quantities of aspartame are enough to sweeten food and drinks, with a considerable calorie saving, useful for those who want to keep the energy intake of the diet under control (you have to smile in front of the many people at the bar who quickly and furiously swallow a couple of pastries, then sweetening the coffee with aspartame to save a few calories).
Aspartame has the great advantage of not significantly altering blood sugar and is therefore well tolerated even by diabetic people, who must necessarily reduce the consumption of traditional sugar. It is also an acaryogenic substance which, unlike saccorose, does not causes tooth decay.
The stability of aspartame is excellent, especially for applications with low H2O content (powdered beverages to be solubilized). This artificial sweetener is also fairly resistant to processes that require heat, such as dairy products, and high or ultra high temperatures. temperatures for short times (especially in the encapsulated form). However, the possibility of hydrolyzing or cyclising to diketopiperazine if exposed to high temperatures for a long time, limits some applications (the "do not cook" warning is given on sweeteners based on aspartame) and makes it contraindicated in pregnancy and breastfeeding ( diketopiperazine is toxic to the fetus).
Safety of use and side effects
In recent years, aspartame has been extensively studied by scientists from all over the world, who have ascertained its safety through experiments on animals and humans. Once ingested, aspartame is rapidly metabolized into its three components: Aspartic acid, Phenylanine and Methanol. These metabolic products have often been the subject of discussion regarding potential toxicity. However, we are talking about substances normally present in the diet; only in rare cases, such as for individuals with phenylketonuria (a rare genetic disease in which phenylalanine is not metabolized) should the use of aspartame be limited. For this reason, sweeteners and other food or dietary products containing aspartame must carry the warning "contains a source of phenylalanine".
Aspartame produces about 10% methanol (a toxic substance) by weight of the ingested dose, which is well below that consumed through the consumption of fruit, vegetables and juices. However, many of the controversies around alleged neurotoxicity of aspartame (balance disturbances, mood disorders, nausea, headache, indistinct vision) concern precisely the release of methanol; the most at risk would be children.
Based on the results of the research conducted, the acceptable daily dose (DGA) established by the JECFA (Joint FAO / WHO Expert Committee on Food Additives) agency is 40mg / kg body weight (FAO = Food and Agriculture Organization; WHO = World Health Organization). Being about 200 times sweeter than sugar, for a 60 kg person an ADI of 40mg / kg is equivalent to 480g of daily sucrose (which would lead to the early onset of various metabolic diseases linked to obesity, such as hyperlipidemia, insulin resistance, cardiovascular problems and increased susceptibility to some cancers).
In food products, aspartame is often indicated with the initials E951. In recent years, in the wake of persistent scientific research concerning the alleged toxicity (which to tell the truth have been repeated, between confirmations and denials, for many years now), the "aspartame has increasingly been replaced by other artificial sweeteners, such as sucralose. Today there is no certainty about the alleged carcenogenicity of aspartame, which continues to be considered a safe sweetener by the FDA (the most important US and world body in charge of regulating food and pharmaceutical products) and by the "EFSA (European Authority for the Food safety).
Other Foods - Sweeteners Acesulfame K Aspartame Sugar beet Sugar cane Sodium cyclamate Dextrose Sweeteners Erythritol Fructose Maltose Mannitol Molasses Saccharin Saccharose Maple syrup Agave syrup Fructose syrup Glucose syrup Sugar sorbitol Articles Stevia Sucralitol sugar SWEETENERS Categories Alcoholic Foods Meat Cereals and derivatives Sweeteners Sweets Offal Fruit Dried fruit Milk and Legumes Oils and Fats Fish and fishery products Salami Spices Vegetables Health recipes Appetizers Bread, Pizza and Brioche First courses Second courses Vegetables and Salads Sweets and Desserts Ice cream and sorbets Syrups, liqueurs and grappas Basic Preparations ---- In the Kitchen with leftovers Carnival recipes Christmas recipes Light diet recipes tici Recipes for the Holidays Recipes for Valentine's Day Vegetarian Recipes Protein Recipes Regional Recipes Vegan Recipes


.jpg)
.jpg)