
The pH is an evaluation parameter of the acidity or basicity of gases and liquids. It is expressed on a scale from 1 to 14, where 7 indicates a condition of neutrality. Values from 1 to 6 are considered acid and values from 8 to 14 are considered basic or alkaline.
The acidifying compound par excellence is hydrogen (H). Not for nothing, pH is the acronym of pondus hydrogenii.
The pH is "potentially" influenced by the "metabolic activity of the organism, therefore by the work of the tissues (for example the muscle), by the hormonal axis, by neurotransmitters, by the nutritional intake with the diet, etc."
In healthy people, however, these are imperceptible alterations, thanks to the intervention of the physiological pH regulation systems. If these were to fail, death would ensue.
For some on the other hand, the fluctuations that tend to lower the pH value, even if buffered quickly - to ensure survival and metabolic homeostasis (state of equilibrium) - in the long term would be responsible for some imbalances.
As we will see, this is not an easily demonstrable theory and the scientific data available today do not support this hypothesis.
Below we will try to shed more light on the subject.
For further information: pH Diet and Fitness atom H2.
Paradoxically, despite having a "very high flammability of the volatile state, if bound to" oxygen (O), the bi-atom gives rise to water (H2O) - which we know to have a suppressing or inhibiting effect on most combustion.
The simplest ways to obtain it, in its bi-atomic form of course, are the solution of metals (for example zinc) in acid substances, or the electrolysis of water - industrial process.
However, in addition to amorphous materials such as certain rocks, hydrogen is also abundant in organic compounds and living organisms; in certain circumstances, it exerts a rather strong acidifying power, but theoretically controllable by the respective physiological mechanisms.
organic liquid of which they are composed, the cells would retain the potential to survive almost perpetually.
So, is acidification the enemy of health and survival? Only if it is pathological, even if different currents of thought - never proven - claim the opposite.
The pH of any element of the organism must fall within what we could define as "normality", punishment, physiological malfunction or even death.
Cellular life itself depends on the electric potential, therefore on the pH, between the nucleus and the cytoplasm - the liquid in which it is immersed. The cytosol is alkaline and positively charged, while the nucleus is acidic and negatively charged. This gap determines the electrical potential necessary for essential biochemical processes.
As mentioned above, to maintain a physiological and homeostatic condition, the blood (or rather the plasma) needs a pH of 7.4 and tolerable fluctuations of ± 0.05 (7.35 - 7.45) are allowed.
We know that the function of the blood is mainly to transport "to" and "from" the tissues. Since any biochemical reaction is influenced by pH, excessively acidifying or alkalizing the plasma would seriously hamper all these processes.
To be clear right away, the healthy organism is perfectly capable of maintaining this condition. Depending on the pH variation, it reacts to fluctuations by releasing alkalizing or acidifying compounds, and expelling "undesirable" factors.
The expulsion occurs mainly (but not only) with two mechanisms:
- lung ventilation with exhaled breath - CO2, volatile ketoacids, ethyl alcohol, etc .;
- renal filtration with urine - all nitrogenous groups;
But what are the factors that can undermine the homeostatic balance of the blood plasma?
In reality, only pathological conditions are able to favor certain imbalances. Diet and training, on the other hand, are part of the physiological conditions that the body is perfectly capable of managing.
", there are acidifying and other alkalizing foods.
But be careful, not all acidic ones are acidifying and vice versa. It may seem strange, but the ability to behave as an acid or as a base depends on the strength of the acid in question and the environment in which it is found.
For example, citric acid often acts as an alkalizer and constitutes an "acidity regulator" also widely used in the food industry. The excess of purines, on the other hand, leads to an increase in uric acid (residual from metabolism). Protein excess also tends to acidification, due to a higher nitrogen residue.
To explain these concepts we would have to do more chemistry lessons, but it is not the subject of this article.
Instead, let's take a "trivial" example.
Let's assume we are introducing and absorbing a certain amount of acidic or acidifying foods, so to speak.
In reality, at the beginning of the intestine (duodenum and jejunum) there is already a very effective tamponade mechanism which excludes the possibility of absorbing excessive quantities of acid molecules. Let alone that it is able to neutralize the hydrochloric acid of the stomach.
So let's pretend that there are conspicuous levels of acid molecules inside the plasma. However, they would not be able to lower the pH, because the organism is perfectly capable of secreting alkaline buffering compounds (bicarbonates) and exploiting the action of alkalizing ions ( such as calcium, potassium and magnesium) which, reacting with acids, facilitate their elimination with urine and respiration, maintaining the physiological pH.
Already from these few lines it is quite clear that - in healthy people - foods, whether acidic or basic, cannot be considered harmful to the pH of the plasma.
urine pH
It is different for the urine which, being a means of expulsion, especially in conditions of poor hydration, can concentrate high levels of acidic or basic compounds.
In the chronic, an excessively acidic pH can favor renal lithiasis, just as a too basic pH is a risk factor for bacterial ascent in the urethra (cystitis, urethritis, etc.).
, a residue of anaerobic glycolysis - necessary for efforts that cannot be supported by aerobic metabolism, therefore of intensity beyond the anaerobic threshold and / or prolonged and / or with insufficient recovery times.
The accumulation of muscle lactic acid causes a reduction in contractile function, as it tends to split into lactate ion and H + ion which hinder normal biochemical processes.
The first defense mechanism of the organism is therefore the displacement of the intracellular acid factors in the extracellular environment and up to the plasma.
Here, too, they can still accumulate by participating in the sensation of metabolic fatigue (with an increase in the respiratory rate and therefore pulmonary) and central (of the nervous system), but there are no alternatives.
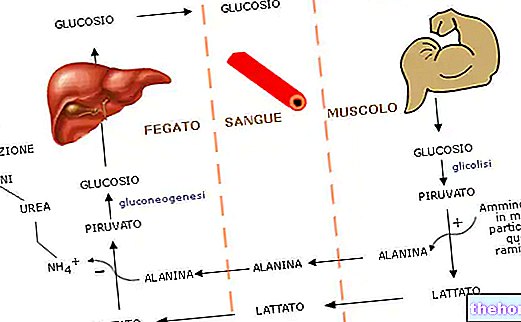
In fact, intracellular lactate must still be released into the blood as soon as possible, because it is here that it can be buffered by bicarbonates or conducted to the liver for neoglucogenesis - production of glucose from scratch - or to other tissues that can use it - such as the cardiac one.
In addition to lactic acid, there are other catabolites due to the practice of intense and / or prolonged motor activity, which are also potentially acidifying; the most important are certainly the ketoacids, which increase above all in the shortage of glucose.
In the healthy organism, however, none of these, not even the most feared ketone bodies, is able to favor alterations in plasma pH such as to compromise the state of health.
it is a pathological condition. If it did, it would undoubtedly have to be treated to avoid dire consequences.However, acidosis cannot arise in healthy people, even if they ate only acidic foods or foods that favor plasma acidity.
So what good would it be to stick to an alkaline diet? Proponents of this system believe that metabolic "tendency" can compromise many physiological processes. We speak of trend and not of significant alteration, because it would be (in theory) of microscopic variations that would remain within the normal range.
Among the unfortunate consequences we mention perhaps the most discussed one, namely the worsening of bone metabolism with insufficient mineralization and a tendency to osteoporosis.
We reiterate that there is no evidence that a potentially acidifying diet can favor this pathological condition, as well as all the others discussed by the promoters of the acid-base diet.
or calcium and the organism results in homeostasis, it would reduce the absorption or increase the excretion of these two minerals; as well as the other way around.
Rather, the imbalances arise due to endocrine, nervous or metabolic compromises of the biochemical signaling pathways.
Then of course, the recommendation is always to take the right dose of foods rich in potassium and magnesium, the main cell and plasma alkalizers. But this goes beyond the concept of an alkaline diet, based on the principle of a healthy and balanced diet in a broad sense.
, a renal or hepatic insufficiency.In these cases, the control of pH takes on a global significance of primary importance, not only for the well-being, but for the survival of the individual.
For further information: Alkaline Diet











.jpg)











