What is Xeljanz - Tofacitinib and what is it used for?
Xeljanz is a medicine used to treat adults with moderate to severe rheumatoid arthritis, a disease that causes inflammation of the joints.
Xeljanz is used together with methotrexate after treatment with one or more medicines known as disease-modifying antirheumatic drugs (DMARDs) have not worked enough or have caused bothersome side effects. It can also be used on its own by patients who cannot take or who are intolerant to methotrexate.
Xeljanz contains the active substance tofacitinib.
How is Xeljanz - Tofacitinib used?
Xeljanz is available as a 5 mg tablet to be taken by mouth twice a day. Treatment may be stopped in patients who develop an infection, as this is a known side effect of the medicine, or in patients with abnormal blood test results. The dose may also be decreased in some patients with reduced kidney or liver function. more information, see package leaflet.
Xeljanz can only be obtained with a prescription. Treatment should be initiated and supervised by a physician experienced in the treatment of rheumatoid arthritis.
How does Xeljanz - Tofacitinib work?
The active substance in Xeljanz, tofacitinib, works by blocking the action of enzymes known as Janus kinase. These enzymes play an important role in the inflammation and joint damage that occurs in rheumatoid arthritis. By blocking their action, tofacitinib helps reduce inflammation and other symptoms of the disease.
What benefit has Xeljanz - Tofacitinib shown during the studies?
Six studies involving more than 4,200 rheumatoid arthritis patients have shown that Xeljanz is effective in reducing joint pain and swelling, improving joint movement and slowing joint damage. Most of the patients in these studies had tried other treatments before and most took Xeljanz with methotrexate.
In one of the studies, where Xeljanz was taken on its own (alone), it was more effective than methotrexate in slowing joint damage and reducing symptoms. In another study, Xeljanz taken alone was more effective than placebo at reducing symptoms such as pain and swelling.
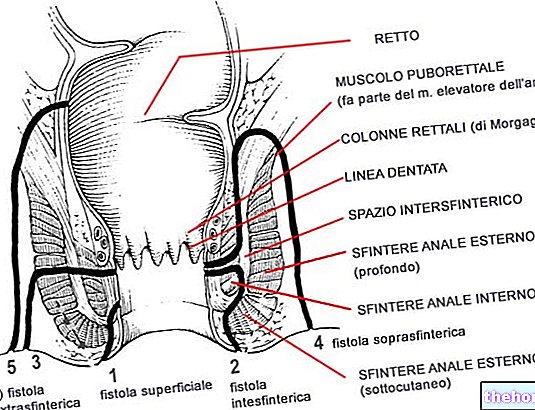
What are the risks associated with Xeljanz - Tofacitinib?
The most common side effects of Xeljanz are headache, nose and throat infection and inflammation, diarrhea, nausea and hypertension (high blood pressure).
The most common serious side effects seen with Xeljanz are serious infections such as pneumonia (lung infection), cellulitis (deep skin tissue infection), herpes zoster (St. Anthony's fire), urinary tract infection, diverticulitis (infection affecting the intestine) and appendicitis (appendix infection), as well as opportunistic infections that can occur in patients with weakened immune systems.
Xeljanz must not be used in patients with active tuberculosis, severe infections or any opportunistic infections. Xeljanz must not be used in patients with severe hepatic impairment and in women who are pregnant or breastfeeding.
For the full list of side effects and limitations, see the package leaflet.
Why has Xeljanz - Tofacitinib been approved?
Several studies have shown that Xeljanz taken with methotrexate is effective in treating rheumatoid arthritis in patients who have previously tried other treatments. Xeljanz is also effective when taken alone.
The most important side effect observed with the medicine is infection and specific recommendations are given to healthcare professionals to reduce this risk. Overall, the risks associated with Xeljanz were similar to those associated with other medicines in its class.
The Agency's Committee for Medicinal Products for Human Use (CHMP) concluded that Xeljanz's benefits are greater than its risks and recommended that it be approved for use in the EU.
What measures are being taken to ensure the safe and effective use of Xeljanz - Tofacitinib?
The company that markets Xeljanz will provide educational material to healthcare professionals and patients to raise awareness of the risks associated with the medicine, in particular the risk of serious infections, and how to treat them.
The recommendations and precautions to be observed by healthcare professionals and patients for Xeljanz to be used safely and effectively have also been reported in the summary of product characteristics and package leaflet.
More information about Xeljanz - Tofacitinib
For the complete version of the Xeljanz EPAR, please consult the Agency's website: ema.europa.eu/Find medicine / Human medicines / European public assessment reports. For more information about Xeljanz therapy, read the package leaflet (included with the EPAR) or contact your doctor or pharmacist.
The information on Xeljanz - Tofacitinib published on this page may be out of date or incomplete. For a correct use of this information, see the Disclaimer and useful information page.