Generality
Nucleotides are the organic molecules that make up the DNA and RNA nucleic acids.
Nucleic acids are biological macromolecules of fundamental importance for the survival of a living organism, and nucleotides are the building blocks of them.
All nucleotides have a general structure that includes three molecular elements: a phosphate group, a pentose (i.e. a 5-carbon sugar) and a nitrogenous base.
In DNA, pentose is deoxyribose; in the RNA, on the other hand, it is ribose.
The presence of deoxyribose in DNA and ribose in RNA represents the main difference between the nucleotides constituting these two nucleic acids.
The second important difference concerns the nitrogenous bases: the nucleotides of DNA and RNA have in common only 3 of the 4 nitrogenous bases associated with them.
What are nucleotides?
Nucleotides are the organic molecules constituting the monomers of DNA and RNA nucleic acids.
According to another definition, nucleotides are the molecular units that make up the nucleic acids DNA and RNA.
Chemical and biological monomers define molecular units which, being arranged in long linear chains, form large molecules (macromolecules), better known as polymers.
General structure
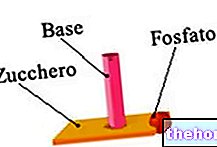
Nucleotides possess a molecular structure that includes three elements:
- A phosphate group, which is a derivative of phosphoric acid;
- A sugar with 5 carbon atoms, that is, a pentose;
- A nitrogenous base, which is an aromatic heterocyclic molecule.
The pentose represents the central element of the nucleotides, as the phosphate group and the nitrogenous base bind to it.

Figure: Elements that make up a generic nucleotide of a nucleic acid. As can be seen, the phosphate group and the nitrogen base bind to the sugar.
The chemical bond that holds the pentose and the phosphate group together is a phosphodiester bond (or phosphodiester bond), while the chemical bond that binds the pentose and the nitrogenous base is an N-glycosidic bond (or N-glycosidic bond. ).
WHICH COALS OF THE PENTOSO ARE INVOLVED IN THE VARIOUS LINKS?
Premise: chemists have thought of numbering the carbons that make up organic molecules, in such a way as to simplify their study and description. Here, then, that the 5 carbons of a pentose become: carbon 1, carbon 2, carbon 3, carbon 4 and carbon 5. The criterion for assigning the numbers is quite complex, therefore we consider it appropriate to leave it out.
Of the 5 carbons that form the pentose of the nucleotides, those involved in the bonds with the nitrogenous base and the phosphate group are, respectively, carbon 1 and carbon 5.
- Pentose carbon 1 → N-glycosidic bond → nitrogen base
- Pentose carbon 5 → phosphodiester bond → phosphate group
NUCLEOTIDES ARE NUCLEOSIDES WITH A PHOSPHATE GROUP

Figure: Structure of a pentose, numbering of its constituent carbons and bonds with nitrogen base and phosphate group.
Without the phosphate group element, nucleotides become nucleosides.
A nucleoside, in fact, is an organic molecule, deriving from the union between a pentose and a nitrogenous base.
This annotation serves to explain some definitions of nucleotides, which state: "nucleotides are nucleosides that have one or more phosphate groups bonded to carbon 5".
Difference between DNA and RNA
The nucleotides of DNA and RNA differ from each other, from the structural point of view.
The main difference lies in the pentose: in DNA, the pentose is deoxyribose; in the RNA, on the other hand, it is ribose.
Deoxyribose and ribose are different for only one atom: in fact, an oxygen atom is missing on carbon 2 of deoxyribose (NB: c "is only a hydrogen), which, on the contrary, is present on carbon 2 of ribose (NB: here, oxygen joins a hydrogen, forming a hydroxyl group (OH).
This difference alone has enormous biological importance: DNA is the genetic patrimony on which the development and adequate functioning of the cells of a living organism depend; RNA, on the other hand, is the biological macromolecule mainly responsible for coding, decoding, regulating and expressing DNA genes.
The other important difference between DNA and RNA nucleotides concerns the nitrogenous bases.
To fully understand this second inequality, it is necessary to take a small step back.


Figure: 5-carbon sugars that make up the nucleotides of RNA (ribose) and DNA (deoxyribose).
Nitrogen bases are molecules of organic nature, which, in nucleic acids, represent the distinctive element of the different types of constituent nucleotides. In fact, in DNA nucleotides as well as in RNA nucleotides, the only variable element is the nitrogenous base. ; the sugar-phosphate group skeleton remains unchanged.
Both in the DNA and in the RNA, the possible nitrogenous bases are 4; therefore the types of nucleotides, for each nucleic acid, are in all 4.
Having said that, returning to the second important difference between the nucleotides of DNA and RNA, these two nucleic acids have in common only 3 out of 4 nitrogenous bases. In this case, adenine, guanine and cytosine are the 3 nitrogenous bases. present in both DNA and RNA; thymine and uracil, on the other hand, are the fourth nitrogenous base of DNA and the fourth base of RNA, respectively.
Therefore, apart from the pentose, the DNA nucleotides and RNA nucleotides are the same for 3 out of 4 types.
Membership classes of nitrogenous bases
Adenine and guanine belong to the class of nitrogenous bases, known as purines. Purines are double ring aromatic heterocyclic compounds.
Thymine, cytosine and uracil, on the other hand, belong to the class of nitrogenous bases, known as pyrimidines. Pyrimidines are single-ring aromatic heterocyclic compounds.
OTHER NAME OF DNA AND RNA NUCLEOTIDES
The nucleotides with deoxyribose sugar, ie the DNA nucleotides, take the alternative name of deoxyribonucleotides, precisely due to the presence of the aforementioned sugar.
For similar reasons, the nucleotides with the sugar ribose, that is the nucleotides of the RNA, take on the alternative name of ribonucleotides.
- Deoxyribonucleotide adenine
- Guanine deoxyribonucleotide
- Deoxyribonucleotide cytosine
- Deoxyribonucleotide thymine
- Ribonucleotide adenine
- Guanine Ribonucleotide
- Cytosine Ribonucleotide
- Uracil ribonucleotide
Organization in nucleic acids
In composing a nucleic acid, the nucleotides organize themselves into long strands, similar to chains.
Each nucleotide forming these long strands binds to the next nucleotide by means of a phosphodiester bond between the carbon 3 of its pentose and the phosphate group of the immediately following nucleotide.
THE EXTREMITIES
The nucleotide strands (or nucleotide strands), which make up nucleic acids, have two ends, known as the 5 "end (read" five end prime ") and 3" end (read "three end prime"). By convention, biologists and geneticists have established that "end 5" represents the head of a strand forming a nucleic acid, while "end 3" represents its tail.
From the chemical point of view, the "5 end" coincides with the phosphate group of the first nucleotide of the chain, while the "3 end" coincides with the hydroxyl group (OH) placed on carbon 3 of the last nucleotide.
It is on the basis of this organization that, in the books of genetics and molecular biology, the nucleotide strands are described as follows: P-5 "→ 3" -OH.
* Note: the letter P indicates the phosphorus atom of the phosphate group.
Biological role
The expression of genes depends on the DNA nucleotide sequence. Genes are more or less long segments of DNA (ie segments of nucleotides), which contain the information essential for the synthesis of proteins. Made up of amino acids, proteins are biological macromolecules, which play a fundamental role in regulating the cellular mechanisms of an organism.
The nucleotide sequence of a given gene specifies the amino acid sequence of the related protein.




.jpg)


















