Active ingredients: Silodosin
Urorec 8 mg hard capsules
Urorec 4 mg hard capsules
Indications Why is Urorec used? What is it for?
What is Urorec
Urorec belongs to a group of medicines called alpha1A-adrenoceptor inhibitors. Urorec is selective for receptors located in the prostate, bladder and urethra. By blocking these receptors, the medicine relaxes the smooth muscle in these tissues. makes it easier to urinate and relieves symptoms.
What is Urorec used for
Urorec is used in adult men to treat urinary symptoms associated with benign enlargement of the prostate (prostatic hyperplasia), such as:
- difficulty starting to urinate,
- feeling that you have not completely emptied your bladder,
- frequent need to urinate, even at night.
Contraindications When Urorec should not be used
Do not take Urorec
if you are allergic to silodosin or any of the other ingredients of this medicine (listed in section 6).
Precautions for use What you need to know before taking Urorec
Talk to your doctor or pharmacist before taking Urorec
- If you are going to have eye surgery due to crystal clouding (cataract surgery), it is important that you tell your eye doctor immediately that you are using or have used Urorec in the past. This is because some patients treated with this type of medicine have experienced a loss of muscle tone in the iris (the colored circular part of the eye) during this procedure. The ophthalmologist will take appropriate precautions regarding the medicines and surgical techniques that will be used. Ask your doctor if it is necessary to postpone or temporarily stop treatment with Urorec in case of cataract surgery.
- If you have fainted in the past or felt dizzy when suddenly standing up, tell your doctor before taking Urorec. When taking Urorec you may experience dizziness on standing up and occasionally fainting, particularly at the start of treatment or if you take Urorec. other medicines that lower blood pressure In this case, sit or lie down immediately until the symptoms disappear and tell your doctor as soon as possible (see also section "Driving and using machines").
- If you have severe liver problems you should not take Urorec as the medicine has not been studied in patients with these conditions.
- If you have kidney problems, ask your doctor for advice. If you have moderate kidney problems, your doctor will start treatment with Urorec with caution and possibly with a reduced dose (see section 3 "Dose"). If you have severe kidney disease you must not take Urorec.
- Since benign enlargement of the prostate and prostate cancer can have the same symptoms, your doctor will check that you do not have prostate cancer before starting treatment with Urorec. Urorec is not a treatment for prostate cancer.
- Treatment with Urorec can lead to abnormal ejaculation (reduction in the amount of semen released during sexual intercourse), which can temporarily impair male fertility. This effect disappears after stopping treatment with Urorec. Tell your doctor if you want to have children.
Children and adolescents
Do not give this medicine to children and adolescents under the age of 18, as there is no indication for this age group.
Interactions Which drugs or foods can modify the effect of Urorec
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
In particular, tell your doctor if you take:
- medicines that lower blood pressure (especially medicines called alpha1-blockers such as prazosin or doxazosin), because there is a potential risk that the effect of these medicines will be increased during treatment with Urorec.
- antifungal medicines (such as ketoconazole or itraconazole), medicines used to control HIV / AIDS infection (such as ritonavir) or medicines used after transplantation to prevent organ rejection (such as cyclosporine), as these medicines may increase the concentration of Urorec in the blood.
- medicines used if you have problems getting or maintaining an erection (such as sildenafil or tadalafil), because concomitant use with Urorec may slightly lower your blood pressure.
- medicines for epilepsy or rifampicin (a medicine used to treat tuberculosis), as the effect of Urorec may be reduced.
Warnings It is important to know that:
Driving and using machines
Do not drive or use machines if you feel faint, dizzy or sleepy or have blurred vision.
Dose, Method and Time of Administration How to use Urorec: Posology
Always use this medicine exactly as your doctor or pharmacist has told you. If in doubt, consult your doctor or pharmacist.
The recommended dose is one Urorec 8 mg capsule per day orally.
Always take the capsule with a meal, preferably at the same time each day. Do not crush or chew the capsule, but swallow it whole, preferably with a glass of water.
Patients with kidney problems
If you have moderate kidney problems, your doctor may prescribe a different dose. For these cases Urorec 4 mg hard capsules are available.
Overdose What to do if you have taken too much Urorec
If you take more Urorec than you should
If you have taken more than one capsule, tell your doctor as soon as possible. If you feel dizzy or faint, tell your doctor immediately.
If you forget to take Urorec
If you forgot to take a capsule earlier, you can take it later on the same day. If it is almost time for your next dose, skip the forgotten dose. Do not take a double dose to make up for a forgotten capsule.
If you stop taking Urorec
If you stop treatment, your symptoms may return.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
Side Effects What are the side effects of Urorec
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Contact your doctor immediately if you notice any of the following allergic reactions: swelling of the face or throat, difficulty in breathing, feeling faint, itchy skin or hives, as the consequences can become serious.
The most common side effect is a reduction in the amount of semen released during sexual intercourse. This effect disappears after stopping treatment with Urorec. Tell your doctor if you want to have children.
Dizziness may occur, including when standing up and occasionally fainting.If you feel faint or dizzy, sit or lie down immediately until the symptoms disappear. If you feel dizzy when standing up or if you faint, tell your doctor as soon as possible.
Urorec can cause complications during cataract surgery (surgery on the eye performed to remedy the clouding of the lens, see section "Warnings and precautions"). It is important that you inform your eye doctor immediately if you are using or have used Urorec in precedence.
Possible side effects are listed below:
Very common side effects (may affect more than 1 in 10 people)
- Abnormal ejaculation (reduction or absence of semen emission during sexual intercourse, see section "Warnings and precautions")
Common side effects (may affect up to 1 in 10 people)
- Dizziness, even on standing up (see also earlier in this paragraph)
- Runny or stuffy nose
- Diarrhea
Uncommon side effects (may affect up to 1 in 100 people)
- Decreased sexual desire
- Nausea
- Dry mouth
- Difficulty getting or maintaining an erection
- Accelerated heart rate
- Symptoms of an allergic skin reaction, such as rash, itching, hives, and drug-induced rash
- Abnormal liver function tests
- Low blood pressure
Rare side effects (may affect up to 1 in 1,000 people)
- Rapid or irregular heartbeats (called palpitations)
- Fainting / loss of consciousness
Very rare side effects (may affect up to 1 in 10,000 people)
- Other allergic reactions with swelling of the face or throat
Not known (frequency cannot be estimated from the available data)
- Flag pupil during cataract surgery (see also earlier in this paragraph)
If it seems to you that there is any effect on your sex life, please tell your doctor.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects you can help provide more information on the safety of this medicine.
Expiry and Retention
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and blister after EXP / EXP. The expiry date refers to the last day of that month.
Do not store above 30 ° C.
Store in the original package to protect from light and moisture.
Do not use this medicine if you notice that the pack is damaged or if it shows signs of tampering.
Do not throw any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. This will help protect the environment.
Composition and pharmaceutical form
What Urorec contains
Urorec 8 mg
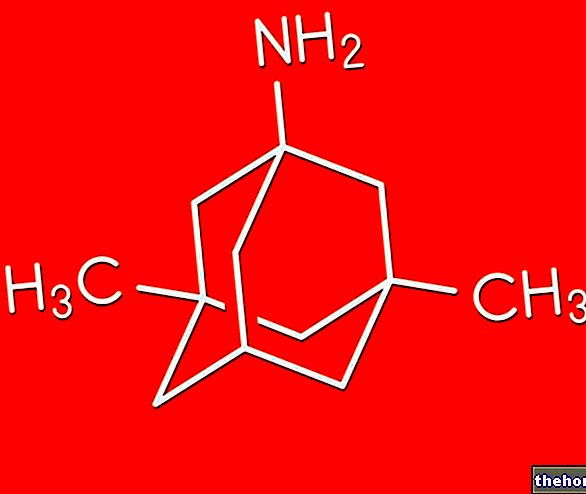
The active ingredient is silodosin. Each capsule contains 8 mg of silodosin.
The other ingredients are pregelatinised maize starch, mannitol (E421), magnesium stearate, sodium lauryl sulfate, gelatin, titanium dioxide (E171).
Urorec 4 mg
The active ingredient is silodosin. Each capsule contains 4 mg of silodosin.
The other ingredients are pregelatinised maize starch, mannitol (E421), magnesium stearate, sodium lauryl sulfate, gelatin, titanium dioxide (E171), yellow iron oxide (E172).
What Urorec looks like and contents of the pack
Urorec 8 mg are white, opaque, hard, gelatin capsules.
Urorec 4 mg are yellow opaque hard, gelatin capsules.
Urorec is available in packs containing 5, 10, 20, 30, 50, 90, 100 capsules. Not all pack sizes may be marketed.
Source Package Leaflet: AIFA (Italian Medicines Agency). Content published in January 2016. The information present may not be up-to-date.
To have access to the most up-to-date version, it is advisable to access the AIFA (Italian Medicines Agency) website. Disclaimer and useful information.
01.0 NAME OF THE MEDICINAL PRODUCT
UROREC 4 MG HARD CAPSULES
02.0 QUALITATIVE AND QUANTITATIVE COMPOSITION
Each hard capsule contains 4 mg of silodosin.
For the full list of excipients, see section 6.1.
03.0 PHARMACEUTICAL FORM
Hard capsule.
Hard gelatin capsule, yellow, opaque, size 3.
04.0 CLINICAL INFORMATION
04.1 Therapeutic indications
Treatment of the signs and symptoms of benign prostatic hyperplasia (BPH) in adult men.
04.2 Posology and method of administration
Dosage
The recommended dose is one Urorec 8 mg capsule per day. For special patient populations, one Urorec 4 mg capsule per day is recommended (see below).
Senior citizens
No dosage adjustment is required in the elderly (see section 5.2).
Renal impairment
In patients with mild renal impairment (CLCR ≥50 to ≤80 mL / min) no dosage adjustment is required.
In patients with moderate renal impairment (CLCR ≥30 to
Hepatic impairment
No dosage adjustment is necessary in patients with mild to moderate hepatic impairment.
Use in patients with severe hepatic impairment is not recommended as no data are available (see sections 4.4 and 5.2).
Pediatric population
There is no indication for a specific use of Urorec in the pediatric population in the authorized indication.
Method of administration
Oral use.
The capsule should be taken with food, every day, preferably at the same time. The capsule should not be crushed or chewed, but should be swallowed whole, preferably with a glass of water.
04.3 Contraindications
Hypersensitivity to the active substance or to any of the excipients listed in section 6.1.
04.4 Special warnings and appropriate precautions for use
Intraoperative Floppy Iris Syndrome (Intraoperative Floppy Iris Syndrome, IFIS)
IFIS (a variant of small pupil syndrome) has been observed during cataract surgery in some patients treated with α1-blockers or previously treated with α1-blockers. This circumstance may increase procedural complications during surgery.
Initiating silodosin therapy in patients awaiting cataract surgery is not recommended. Discontinuation of α1-blocker treatment 1-2 weeks prior to cataract surgery has been recommended, but the benefits and duration of discontinuing therapy prior to cataract surgery have not yet been established.
During the preoperative evaluation, eye surgeons and the entire team should consider whether patients awaiting cataract surgery are being treated or have been treated with silodosin, in order to ensure that appropriate measures are available to address IFIS during "intervention.
Orthostatic effects
The incidence of orthostatic effects with silodosin is very low. However, a decrease in blood pressure may occur in individual patients, which rarely can be a cause of syncope. At the first symptoms of orthostatic hypotension (such as postural dizziness), the patient should sit or lie down until symptoms disappear In patients with orthostatic hypotension, treatment with silodosin is not recommended.
Renal impairment
The use of silodosin in patients with severe renal impairment (CLCR
Hepatic impairment
Since no data are available, the use of silodosin is not recommended in patients with severe hepatic impairment (see sections 4.2 and 5.2).
Prostate cancer
Since BPH and prostate cancer can have the same symptoms and can coexist, patients considered to have BPH should be evaluated before starting therapy with silodosin to rule out the presence of prostate cancer. then, at regular intervals, digital rectal examination and, if necessary, prostate specific antigen (PSA) measurement should be performed.
Treatment with silodosin results in reduced or no ejaculation during orgasm, which can temporarily impair male fertility. The effect disappears after discontinuation of silodosin treatment (see section 4.8).
04.5 Interactions with other medicinal products and other forms of interaction
Silodosin is extensively metabolised, mainly via CYP3A4, alcohol dehydrogenase and UGT2B7. Silodosin is also a substrate of P-glycoprotein. Substances that inhibit (such as ketoconazole, itraconazole, ritonavir or cyclosporine) or induce (such as rifampicin, barbiturates, carbamazepine, phenytoin) these enzymes and transporters may affect the plasma concentrations of silodosin and its metabolite active.
Alpha blockers
There is no adequate information on the safety of use of silodosin in combination with other α-adrenoceptor antagonists. Therefore, concomitant use of other α-adrenoceptor antagonists is not recommended.
CYP3A4 inhibitors
In an interaction study, a 3.7-fold increase in the maximum plasma concentration of silodosin and a 3.1-fold increase in silodosin exposure (ie AUC) were observed with co-administration of a potent inhibitor of silodosin. CYP3A4 (ketoconazole 400 mg). Concomitant use of potent CYP3A4 inhibitors (such as ketoconazole, itraconazole, ritonavir or cyclosporine) is not recommended.
When silodosin was co-administered with a moderate potency CYP3A4 inhibitor such as diltiazem, an increase in silodosin AUC of approximately 30% was observed, while Cmax and half-life were not affected. This alteration is not clinically relevant and no dosage adjustment is necessary.
PDE-5 inhibitors
Minimal pharmacodynamic interactions have been observed between silodosin and maximum doses of sildenafil or tadalafil. In a placebo-controlled study in 24 subjects aged 45 to 78 years treated with silodosin, co-administration of sildenafil 100 mg or tadalafil 20 mg did not induce clinically significant mean reductions in systolic or diastolic blood pressure as demonstrated. from the orthostatic test (standing versus supine position). In subjects over 65 years of age, the mean reduction at various times ranged from 5 to 15 mmHg (systolic blood pressure) and from 0 to 10 mmHg (diastolic blood pressure). Positive orthostatic tests were only slightly more frequent in case of joint administration; however, there were no episodes of symptomatic orthostatic hypotension or dizziness. Patients treated with PDE-5 inhibitors in conjunction with silodosin should be monitored for possible adverse reactions.
Antihypertensives
As part of the clinical trial program, many patients have been co-treated with antihypertensives (mainly with renin-angiotensin-acting agents, beta-blockers, calcium channel blockers and diuretics), without an increased incidence. of orthostatic hypotension. Nevertheless, caution should be used when initiating concomitant use with antihypertensives and patients should be monitored for possible adverse reactions.
Digoxin
Steady-state levels of digoxin, a substrate of P-glycoprotein, were not significantly changed when co-administered with silodosin 8 mg once daily. No dosage adjustment is necessary.
04.6 Pregnancy and breastfeeding
Pregnancy and breastfeeding
Not relevant as silodosin is intended for male patients only.
Fertility
Cases of ejaculation with reduced or no semen emission (see section 4.8) due to the pharmacodynamic properties of silodosin have been reported during treatment with silodosin. Before starting treatment, the patient should be informed of this possible effect, which temporarily impairs male fertility.
04.7 Effects on ability to drive and use machines
Urorec has no or negligible influence on the ability to drive or use machines. Patients should be informed of the possible occurrence of symptoms related to postural hypotension (such as dizziness) and advised to use caution while driving and operating machinery until they are aware of the possible effects of silodosin on their body.
04.8 Undesirable effects
Summary of the safety profile
The safety of silodosin was evaluated in four phase II-III double-blind controlled clinical trials (with 931 patients treated with silodosin 8 mg once daily and 733 patients treated with placebo) and in two long-term open-label studies. In total, 1,581 patients received silodosin at a dose of 8 mg once daily, including 961 patients exposed for at least 6 months and 384 patients exposed for 1 year.
The most frequent adverse reactions reported with silodosin in placebo-controlled clinical trials and during long-term use were ejaculation disorders such as retrograde ejaculation and anejaculation (reduced or absent ejaculate volume), with a frequency of 23%. This may temporarily impair male fertility. This effect is reversible within a few days of stopping treatment (see section 4.4).
Table of adverse reactions
In the table below, adverse reactions observed in all clinical studies and from post-marketing experience worldwide for which a reasonable causal relationship has been established are listed by MedDRA system organ class and by frequency: very common (≥1 / 10), common (≥1 / 100,
1 - Adverse reactions reported through spontaneous reporting based on post-marketing experience worldwide (frequencies calculated based on events reported in phase I-IV clinical trials and non-interventional studies).
Description of selected adverse reactions
Orthostatic hypotension
The incidence of orthostatic hypotension in placebo-controlled clinical trials was 1.2% with silodosin and 1.0% with placebo. Orthostatic hypotension may occasionally cause syncope (see section 4.4).
Intraoperative Floppy Iris Syndrome (IFIS)
IFIS has been observed during cataract surgery (see section 4.4).
Reporting of suspected adverse reactions
Reporting of suspected adverse reactions occurring after authorization of the medicinal product is important as it allows continuous monitoring of the benefit / risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system.
04.9 Overdose
Silodosin has been evaluated up to maximum doses of 48 mg / day in healthy male subjects. Postural hypotension is the dose-limiting adverse reaction. In case of recent intake, consider inducing vomiting or performing gastric lavage. If silodosin overdose causes hypotension, cardiovascular support should be provided. Cardiovascular support is unlikely. dialysis has a significant benefit, as silodosin is highly protein bound (96.6%).
05.0 PHARMACOLOGICAL PROPERTIES
05.1 Pharmacodynamic properties
Pharmacotherapeutic group: Urologicals, alpha-adrenoceptor antagonists, ATC code: G04CA04.
Mechanism of action
Silodosin is highly selective for α1A-adrenoceptors located mainly in the human prostate, bottom and neck of the urinary bladder, prostatic capsule and prostatic urethra. The blocking of these α1A-adrenoceptors induces relaxation of the smooth muscle of these tissues, with consequent reduction of the resistance of the bladder outflow tract, without compromising the contractility of the detrusor smooth muscle. This results in an improvement in the symptoms of the lower urinary tract (lower urinary tract symptoms, LUTS) related to filling (irritative) and emptying (obstructive), which are associated with benign prostatic hyperplasia.
Silodosin has a markedly lower affinity for α1B-adrenoceptors located mainly in the cardiovascular system. It has been demonstrated in vitro that the α1A: α1B bond ratio of silodosin (162: 1) is extremely high.
Clinical efficacy and safety
In a phase II dose-setting, double-blind, placebo-controlled clinical study conducted with silodosin 4 or 8 mg once daily, a more marked improvement in symptom score was observed.American Urologic Association (AUA) with 8 mg silodosin (-6.8 ± 5.8, n = 90; p = 0.0018) and 4 mg silodosin (-5.7 ± 5.5, n = 88; p = 0.0355 ) compared to placebo (-4.0 ± 5.5, n = 83).
More than 800 patients with moderate to severe BPH symptoms (International Prostate Symptom Score, IPSS, baseline ≥13) were treated with silodosin 8 mg once daily in two placebo-controlled phase III clinical studies conducted in the United States and in one placebo- and active comparator-controlled clinical study. in Europe. In all studies, patients who failed to respond to placebo in a 4-week placebo run-in phase were randomized to receive study treatment. Across all studies, a more marked reduction in both filling (irritative) and voiding (obstructive) symptoms due to BPH was observed in patients treated with silodosin compared to placebo, measured after 12 weeks of treatment. Data observed in the intent-to-treat populations of each study are shown below:
* pvs placebo; ° p = 0.002 vs placebo
In the active comparator controlled clinical study conducted in Europe, silodosin 8 mg once daily was shown to be non-inferior in efficacy to tamsulosin 0.4 mg once daily: the adjusted mean difference (95% CI) in the IPSS total score across treatments of the per-protocol population was 0.4 (-0.4 to 1.1). The responder rate (i.e. improvement in IPSS total score by at least 25%) was significantly greater in the silodosin (68%) and tamsulosin (65%) groups compared to the placebo (53%) group.
In the open-label, long-term extension phase of these controlled trials, in which patients were treated with silodosin for up to 1 year, silodosin-induced symptom improvement at week 12 of treatment was maintained for 1 year.
No significant reductions in supine blood pressure were observed in all clinical studies with silodosin.
Doses of 8 mg and 24 mg per day of silodosin had no statistically significant effect on ECG intervals or cardiac repolarization compared to placebo.
Pediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with Urorec in all subsets of the pediatric population in BPH (see section 4.2 for information on pediatric use).
05.2 Pharmacokinetic properties
The pharmacokinetics of silodosin and its major metabolites were examined in adult male subjects with and without BPH after single and multiple dosing, with doses ranging from 0.1 mg to 48 mg per day. The pharmacokinetics of silodosin are linear over this dose range.
The exposure to the major metabolite in plasma, silodosin glucuronide (KMD-3213G), at steady state is approximately 3 times the exposure to the parent substance. Silodosin and its glucuronide reach steady state after 3 days and 5 days of treatment, respectively.
Absorption
Orally administered silodosin is well absorbed and absorption is dose proportional. Absolute bioavailability is approximately 32%.
In a studio in vitro conducted with Caco-2 cells it has been shown that silodosin is a substrate of P-glycoprotein.
Food reduces Cmax by approximately 30%, increases Tmax by approximately 1 hour and has limited effects on AUC.
In healthy male subjects, representative age of the patients (n = 16, mean age 55 ± 8 years), following administration of 8 mg once daily for 7 days immediately after breakfast, there were following pharmacokinetic parameters: Cmax 87 ± 51 ng / ml (SD), Tmax 2.5 hours (range 1.0-3.0), AUC 433 ± 286 ng • h / ml.
Distribution
Silodosin has a volume of distribution of 0.81 l / kg and is 96.6% bound to plasma proteins. It does not distribute in blood cells.
The protein binding of silodosin glucuronide is 91%.
Biotransformation
Silodosin is extensively metabolised via glucuronidation (UGT2B7), alcohol dehydrogenase, aldehyde dehydrogenase and oxidation, mainly by CYP3A4. The main metabolite in plasma, the glucuronic acid conjugate of silodosin (KMD-3213G), which has been shown to be active in vitro, has a prolonged half-life (approximately 24 hours) and reaches plasma concentrations approximately four times higher than silodosin concentrations. in vitro indicate that silodosin does not have the potential to inhibit or induce the cytochrome P450 enzyme system.
Elimination
After oral administration of 14C-labeled silodosin, recovery of radioactivity after 7 days was approximately 33.5% in urine and 54.9% in faeces. Total clearance of silodosin was approximately 0.28 L / h / kg. Silodosin is mainly excreted in the form of metabolites, minimal amounts of the unchanged substance are recovered in the urine. The terminal half-life of silodosin and its glucuronide is approximately 11 hours and 18 hours, respectively.
Special patient populations
Senior citizens
Exposure to silodosin and its major metabolites does not vary significantly with age, even in patients over 75 years of age.
Pediatric population
Silodosin has not been studied in patients under 18 years of age.
Hepatic impairment
In a single dose study, the pharmacokinetics of silodosin were not altered in nine patients with moderate hepatic impairment (Child-Pugh score between 7 and 9) compared to nine healthy volunteers. The results of this study should be interpreted with caution, as the enrolled patients had normal biochemical values, indicating normal metabolic function, and were classified as having moderate hepatic impairment, based on the presence of ascites and hepatic encephalopathy.
The pharmacokinetics of silodosin have not been studied in patients with severe hepatic impairment.
Renal impairment
In a single dose study, silodosin (unbound) exposure in patients with mild (n = 8) and moderate (n = 8) renal impairment experienced, on average, an increase in Cmax (1.6-fold). and AUC (1.7-fold) compared to patients with normal renal function (n = 8). In subjects with severe renal impairment (n = 5) the increase in exposure was 2.2-fold for Cmax and 3.7 times for the AUC. Exposure to the major metabolites, silodosin glucuronide and KMD-3293, was also increased.
Monitoring of plasma levels in a phase III clinical study showed that total silodosin levels after 4 weeks of treatment did not change in patients with mild impairment (n = 70) compared to patients with normal renal function (n = 155), while they doubled on average in patients with moderate impairment (n = 7).
A review of the safety data obtained in patients enrolled in all clinical studies does not indicate that mild renal impairment (n = 487) carries an additional safety risk during treatment with silodosin (such as increased dizziness or orthostatic hypotension) compared to patients with normal renal function (n = 955). Consequently, no dose adjustment is required in patients with mild renal impairment. Since there is only limited experience in patients with moderate renal impairment (n = 35), a reduced starting dose of 4 mg Administration of Urorec in patients with severe renal impairment is not recommended.
05.3 Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology and carcinogenic, mutagenic and teratogenic potential. Effects in animals (affecting the thyroid gland in rodents) were observed only at exposures considered sufficiently in excess of the maximum human exposure, indicating little relevance to clinical use.
Infertility was observed in male rats from exposures approximately double the exposure at the maximum recommended human dose. The observed effect was reversible.
06.0 PHARMACEUTICAL INFORMATION
06.1 Excipients
Capsule contents
Pregelatinised starch (maize)
Mannitol (E421)
Magnesium stearate
Sodium lauryl sulfate
Capsule shell
Jelly
Titanium dioxide (E171)
Yellow iron oxide (E172)
06.2 Incompatibility
Not relevant.
06.3 Period of validity
3 years.
06.4 Special precautions for storage
Do not store above 30 ° C.
Store in the original package to protect from light and moisture.
06.5 Nature of the immediate packaging and contents of the package
The capsules are supplied in PVC / PVDC / aluminum foil blisters, packed in cartons.
Packs of 5, 10, 20, 30, 50, 90, 100 capsules.
Not all pack sizes may be marketed.
06.6 Instructions for use and handling
No special instructions.
07.0 MARKETING AUTHORIZATION HOLDER
Recordati Ireland Ltd.
Raheens East
Ringaskiddy Co. Cork
Ireland
08.0 MARKETING AUTHORIZATION NUMBER
EU / 1/09/608/001
EU / 1/09/608/002
EU / 1/09/608/003
EU / 1/09/608/004
EU / 1/09/608/005
EU / 1/09/608/006
EU / 1/09/608/007
039789019
039789021
039789033
039789045
039789058
039789060
039789072
09.0 DATE OF FIRST AUTHORIZATION OR RENEWAL OF THE AUTHORIZATION
Date of first authorization: 29/01/2010
10.0 DATE OF REVISION OF THE TEXT
D.CCE September 2014