Active ingredients: Ramipril
QUARK 2.5 mg tablets
QUARK 5 mg tablets
QUARK 10 mg tablets
Indications Why is Quark used? What is it for?
QUARK contains a medicine called ramipril which belongs to a group of medicines called ACE inhibitors (Angiotensin Converting Enzyme Inhibitors).
QUARK acts:
- By decreasing the body's production of substances that can cause blood pressure to rise
- Relaxing and widening your blood vessels
- Making it easier for your heart to pump blood around your body.
QUARK can be used:
- To treat high blood pressure (hypertension)
- To reduce the risk of a heart attack or stroke
- To reduce the risk or delay the worsening of kidney problems (whether or not you have diabetes)
- To treat your heart when it cannot pump enough blood to the rest of the body (heart failure)
- As a treatment after a heart attack (myocardial infarction) when associated with heart failure.
Contraindications When Quark should not be used
Do not take QUARK:
- If you are allergic (hypersensitive) to ramipril, other ACE inhibitors or any of the other ingredients of QUARK listed in section 6. Signs of an allergic reaction may be skin rash, difficulty swallowing or breathing, swelling of the lips, face, in the throat or tongue
- If you have ever had a severe allergic reaction called 'angioedema'. These signs include itching, rash (hives), red spots on the hands, feet and throat, swelling of the throat and tongue, swelling around the eyes and lips, difficulty in breathing and swallowing.
- If you are on dialysis or have some other type of blood filtration. Depending on the machinery being used, QUARK may not be suitable for you
- If you have kidney problems due to insufficient blood supply to the kidney (renal artery stenosis)
- In the last 6 months of pregnancy (see section under "Pregnancy and breastfeeding")
- If your blood pressure is excessively low or unstable. Your doctor will need to make this assessment
- If you have diabetes or impaired kidney function and you are being treated with a blood pressure lowering medicine containing aliskiren.
Do not take QUARK if any of the above conditions apply. If you are not sure, ask your doctor before taking QUARK.
Precautions for use What you need to know before taking Quark
Take special care with QUARK
Check with your doctor or pharmacist before taking QUARK:
- If you have heart, liver or kidney problems
- If you have lost a lot of salts or body fluids (due to being unwell such as vomiting, diarrhea, excessive sweating, or following a low-salt diet, or from taking oral diuretics for a long time or having underwent dialysis)
- If you are about to undergo treatment to reduce allergy to bee or wasp stings (desensitization)
- If you are going to have anesthesia. This can be given for surgery or dental work. You may need to stop taking QUARK the day before, ask your doctor for advice.
- If you have a high amount of potassium in your blood (shown in a blood test)
- If you have a vascular collagen disease such as scleroderma or systemic lupus erythematosus.
- You should tell your doctor if you think you are pregnant (or if there is a possibility of becoming pregnant). QUARK is not recommended in the first three months of pregnancy and may cause serious harm to the baby if taken after three months of pregnancy (see section under "Pregnancy and breastfeeding").
- If you are taking any of the following medicines used to treat high blood pressure: - an "angiotensin II receptor antagonist" (AIIRA) (also known as sartans - for example valsartan, telmisartan, irbesartan), particularly if you have kidney problems related to diabetes - aliskiren
- Your doctor may check your kidney function, blood pressure, and the amount of electrolytes (such as potassium) in your blood at regular intervals.
Children
The use of QUARK is not recommended in children and adolescents below 18 years of age because the safety and efficacy of QUARK in children have not yet been established.
If any of the above apply to you (or you are not sure), ask your doctor before taking QUARK.
Interactions Which drugs or foods can modify the Quark effect
Tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription (including herbal medicines). This is because QUARK can affect the way some other medicines work. Also some medicines can affect the way QUARK works.
Tell your doctor if you are taking any of the following medicines. These medicines can interfere with QUARK by altering its action:
- Medicines used to relieve pain and inflammation (e.g. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) such as ibuprofen, indomethacin, aspirin)
- Medicines used to treat low blood pressure, shock, heart failure, asthma or allergies such as ephedrine, noradrenaline or adrenaline. Your doctor will need to check your blood pressure.
Tell your doctor if you are taking any of the following medicines. These medicines, when taken with QUARK, can increase the likelihood of side effects:
- Medicines used to relieve pain and inflammation (e.g. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) such as ibuprofen, indomethacin, aspirin)
- Medicines to treat cancer (chemotherapy)
- Medicines to avoid organ rejection after transplantation such as cyclosporine
- Diuretics such as furosemide
- Medicines that can increase the amount of potassium in the blood such as spironolactone, triamterene, amiloride, potassium salts and heparin (used to thin the blood)
- Steroid medicines for the treatment of inflammation such as prednisolone
- Allopurinol (used to lower the uric acid content in the blood)
- Procainamide (for heart beat problems).
Tell your doctor if you are taking any of the following medicines. The way these medicines work can be affected by QUARK:
- Medicines for diabetes such as oral hypoglycaemics and insulin. QUARK may lower the amount of sugar in your blood. Check your blood sugar carefully when taking QUARK.
- Lithium (for psychiatric problems). QUARK can increase the amount of lithium in the blood. The level of lithium in your blood should be carefully checked by your doctor.
- Your doctor may need to change your dose and / or take other precautions.
If you are taking an angiotensin II receptor antagonist (AIIRA) or aliskiren (see also information under "Before you take QUARK" and "Take special care with QUARK".
If any of the above apply to you (or you are not sure), ask your doctor before taking QUARK.
Taking QUARK with food and alcohol
- Drinking alcoholic beverages together with QUARK may cause you to feel dizzy or lightheaded. If you want to know how much alcohol to drink while you are taking QUARK, discuss this with your doctor, as drugs used to lower blood pressure and alcohol can have additive effects.
- QUARK can be taken with or between meals.
Warnings It is important to know that:
Pregnancy and breastfeeding
Pregnancy
You should tell your doctor if you think you are pregnant (or if there is a possibility of becoming pregnant). You should not take QUARK in the first 12 weeks of pregnancy and you should not take it at all after the thirteenth week as use during pregnancy may be harmful to the baby. Tell your doctor immediately if you are pregnant while on Quark. In advance of to a planned pregnancy, a switch to an appropriate alternative treatment should be made.
Feeding time
You must not take QUARK if you are breastfeeding. Ask your doctor or pharmacist before taking any medicine
Driving and using machines
You may feel dizzy while taking QUARK. This is more likely when you have just started Quark or just increased your dose. If this happens, do not drive or use any tools or machines.
Dose, Method and Time of Administration How to use Quark: Dosage
Always take QUARK exactly as your doctor has told you. You should seek the advice of your doctor or pharmacist if you are not sure.
Taking this medicine
- Take this medicine by mouth at the same time of the day every day.
- Swallow the tablets whole with liquid.
- Do not break the tablets or chew them.
How much do you have to take
Treatment of high blood pressure
- The usual starting dose is 1.25 mg or 2.5 mg once a day.
- Your doctor will adjust the amount you take until your blood pressure is under control.
- The maximum daily dose is 10 mg.
- If you are already taking diuretics, your doctor may stop or reduce the amount before starting treatment with QUARK.
To reduce the risk of a heart attack or stroke
- The starting dose is 2.5 mg once a day.
- Your doctor may decide to increase the dosage you take
- The usual dose is 10 mg once a day.
Treatment to reduce or prevent worsening of kidney problems
- You may be given a starting dose of 1.25 mg or 2.5 mg once a day
- Your doctor will adjust the amount you are taking.
- The usual dose is 5 mg or 10 mg once a day.
Treatment of heart failure
- The usual starting dose is 1.25 mg once a day.
- Your doctor will adjust the amount you take.
- The maximum dose is 10 mg per day. It is preferable to divide the dose into two daily administrations.
Treatment after a heart attack
- The usual starting dose is 1.25 mg once daily to 2.5 mg twice daily.
- Your doctor will adjust the amount you take.
- The usual dose is 10 mg per day. It is preferable to divide the dose into two daily administrations.
Senior citizens
Your doctor will reduce the starting dose and adjust your treatment more slowly.
If you forget to take QUARK
- If you miss a dose, take your normal dose when it is time for it.
- Do not take a double dose to make up for a forgotten tablet. If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
Overdose What to do if you have taken too much Quark
If you take more QUARK than you should
Tell your doctor or go to the emergency room of the nearest hospital. Do not drive to the hospital, have someone accompany you or call an ambulance. Take the box of medicine with you. This is because your doctor needs to know what you have hired.
Side Effects What are the side effects of Quark
Like all medicines, QUARK can cause side effects, although not everybody gets them.
Stop taking QUARK and see your doctor immediately if you notice any serious side effects - you may need urgent medical treatment:
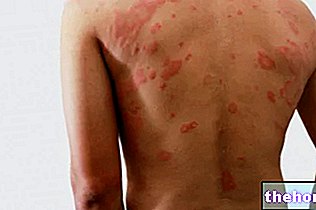
- Swelling of the face, lips or throat that make it difficult to swallow or breathe, as well as itching or rash. This could be a sign of a severe allergic reaction to QUARK.
- Severe skin reactions including rash, mouth ulcers, worsening of a pre-existing skin condition, redness, blistering and peeling of the skin (such as Stevens-Johnson Syndrome, toxic epidermal necrolysis or erythema multiforme).
Tell your doctor immediately if you experience:
- Faster heart rate, irregular or strengthened heartbeat (palpitations), chest pain, tightness in the chest, or more serious problems including heart attack and stroke
- Shortness of breath or cough. These can be signs of lung problems
- Bruising easier, bleeding longer than normal, any signs of bleeding (e.g. bleeding gums) purple spots on the skin or easier onset of infections, throat irritation and fever, feeling tired, weakness, dizziness or pale skin . These can be signs of blood or bone marrow problems
- Severe stomach pain which can extend to the back. This can be a sign of pancreatitis (inflammation of the pancreas)
- Fever, chills, tiredness, loss of appetite, stomach pain, feeling sick, yellowing of the skin or eyes (jaundice). These may be signs of liver problems such as hepatitis (inflammation of the liver) or liver damage. .
Other side effects include:
Tell your doctor if any of the conditions described below become severe or persist for longer than a few days:
Common (affecting less than 1 patient in every 10 patients on therapy)
- Headache or tired feeling
- Feeling dizzy. This is more likely to happen when QUARK therapy has just started or the dose has just been increased
- Weakness, hypotension (unusually low blood pressure), especially when standing or getting up quickly
- Irritating dry cough, sinus inflammation (sinusitis) or bronchitis, shortness of breath
- Pain in the stomach or intestines, diarrhea, indigestion, feeling unwell or being unwell
- Skin rash with or without lumps
- Chest pains
- Muscle cramps or aches
- Blood tests show a higher than normal potassium level.
Uncommon (affecting less than 1 patient in every 100 patients on therapy)
- Balance problems (dizziness)
- Itching and unusual skin sensations such as numbness, tingling, burning, stinging or rubbing (paraesthesia)
- Loss or change in taste
- Sleep problems
- Depressed mood, anxiety, more nervousness than usual or irritability
- Stuffed nose, difficulty breathing or worsening of asthma
- Swelling of the "gut called" intestinal angioedema "and presenting with symptoms such as abdominal pain, vomiting and diarrhea
- Heartburn, constipation or dry mouth
- Increased amount of urine during the day
- More sweating than usual
- Loss or decreased appetite (anorexia)
- Fast or irregular heartbeat.
- Swollen arms and legs. This may be a sign that your body is holding onto more water than usual
- Flushes
- Blurred vision
- Pain in the joints
- Fever
- Impotence in men, reduced sexual desire in men and women
- An increase in the number of white blood cells (eosinophilia) found in blood tests
- Changes in the function of the liver, pancreas or kidneys shown in blood tests.
Rare (affecting less than 1 patient in every 1000 patients on therapy)
- Feeling faint or confused
- Swollen and red tongue
- Severe flaking or peeling of the skin, itchy, pustular rash
- Nail problems (such as loosening or separation of the nail from its place)
- Skin rash or bruising
- Spots on the skin and cold extremities
- Red, swollen or watery or itchy eyes
- Disturbed hearing and ringing in the ear
- Feeling of weakness
- Decrease in the number of red, white blood cells and platelets in the blood or in the concentration of hemoglobin, shown in blood tests.
Very rare (affecting less than 1 patient in 10,000 patients on therapy)
- Increased awareness of the sun.
Other side effects found:
Tell your doctor if any of the conditions described below become severe or persist for longer than a few days.
- Difficulty concentrating
- Swelling in the mouth
- Blood tests showing too few blood cells
- Blood tests showing low blood sodium
- Fingers and toes that change color when they get cold and that tingle or hurt when heated (Raynaud's phenomenon)
- Breast enlargement in men
- Slowed or altered reactions
- Burning sensation
- Change in the perception of odors
- Hair loss
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. Undesirable effects can also be reported directly through the national reporting system at the address www.agenziafarmaco.gov.it/it/responsabili. By reporting side effects you can help provide more information on the safety of this medicine. "
Expiry and Retention
Expiry: see the expiry date indicated on the package. The expiry date indicated refers to the product in intact packaging, correctly stored.
WARNING: do not use the medicine after the expiry date indicated on the package.
Keep this medicine out of the reach and sight of children.
This medicinal product does not require any special storage temperatures.
Other_information "> Other information
What QUARK contains
Quark 2.5 mg tablets
One breakable tablet contains:
Active ingredient: ramipril 2.5 mg.
Excipients: hypromellose, pregelatinised maize starch, microcrystalline cellulose, sodium stearyl fumarate, yellow iron oxide E 172.
Quark 5 mg tablets
One breakable tablet contains:
Active ingredient: ramipril 5 mg.
Excipients: hypromellose, pregelatinised maize starch, microcrystalline cellulose, sodium stearyl fumarate, red iron oxide E 172.
Quark 10 mg tablets
One breakable tablet contains:
Active ingredient: ramipril 10 mg.
Excipients: hypromellose, pregelatinised maize starch, microcrystalline cellulose, sodium stearyl fumarate.
Description of what QUARK looks like and contents of the pack
Tablets.
Quark 2.5 mg tablets
Divisible tablets. Box of 28 tablets.
Quark 5 mg tablets
Divisible tablets. Box of 14 tablets.
Quark 10 mg tablets
Divisible tablets. Box of 28 tablets.
Source Package Leaflet: AIFA (Italian Medicines Agency). Content published in January 2016. The information present may not be up-to-date.
To have access to the most up-to-date version, it is advisable to access the AIFA (Italian Medicines Agency) website. Disclaimer and useful information.
01.0 NAME OF THE MEDICINAL PRODUCT -
QUARK TABLETS
02.0 QUALITATIVE AND QUANTITATIVE COMPOSITION -
Quark 2.5 mg tablets
One breakable tablet contains:
active principle: ramipril 2.5 mg.
Quark 5 mg tablets
One breakable tablet contains:
active principle: ramipril 5 mg.
Quark 10 mg tablets
One breakable tablet contains:
active principle: ramipril 10 mg.
For the full list of excipients, see section 6.1.
03.0 PHARMACEUTICAL FORM -
Tablets.
04.0 CLINICAL INFORMATION -
04.1 Therapeutic indications -
- Treatment of hypertension.
- Cardiovascular prevention: reduction of cardiovascular morbidity and mortality in patients with:
• established atherothrombotic cardiovascular diseases (previous coronary heart disease or stroke, or peripheral vascular disease) or
• diabetes with at least one cardiovascular risk factor (see section 5.1)
- Treatment of kidney diseases:
• Incipient diabetic glomerular nephropathy, defined by the presence of microalbuminuria
• Overt diabetic glomerular nephropathy, defined by macroproteinuria in patients with at least one cardiovascular risk factor (see section 5.1)
• Overt glomerular non-diabetic nephropathy defined by macroproteinuria ≥ 3g / day (see section 5.1).
- Treatment of symptomatic heart failure.
- Secondary prevention after acute myocardial infarction: reduction of mortality after the acute phase of myocardial infarction in patients with clinical signs of heart failure when started 48 hours after the onset of acute myocardial infarction
04.2 Posology and method of administration -
Oral use.
It is recommended that QUARK be taken at the same time each day.
QUARK can be taken before, during or after meals, because food intake does not change its bioavailability (see section 5.2).
QUARK must be swallowed with a liquid and must not be chewed or crumbled.
Adults
Patients being treated with a diuretic
Hypotension may occur after initiation of treatment with QUARK and is more likely in patients treated concomitantly with a diuretic. Caution is therefore recommended for these patients as they may be depleted in plasma volume and / or salts.
The diuretic should be discontinued, if possible, 2 to 3 days prior to initiation of QUARK therapy (see section 4.4).
In hypertensive patients in whom the diuretic has not been discontinued, therapy with QUARK should be initiated with a dose of 1.25 mg. Renal function and serum potassium should be monitored. The subsequent dosage of QUARK must be adjusted according to the desired blood pressure value.
Hypertension
The dose should be individualized according to the patient profile (see section 4.4) and blood pressure control.
QUARK can be used alone or in combination with other classes of antihypertensive drugs (see sections 4.3, 4.4, 4.5 and 5.1).
Initial dose
Treatment with QUARK should be started gradually, with a recommended starting dose of 2.5 mg per day.
Patients with an overactivation of the renin-angiotensin-aldosterone system may have an excessive drop in blood pressure after taking the starting dose. For these patients, a starting dose of 1.25 mg is recommended, and starting treatment under medical supervision (see section 4.4).
Titration and maintenance dose
The dose can be doubled at intervals of 2-4 weeks in order to progressively reach the required blood pressure value; the maximum dose of QUARK is 10 mg per day. The dose is usually taken once a day.
Cardiovascular prevention
Initial dose
The recommended starting dose is 2.5 mg of QUARK once a day.
Titration and maintenance dose
The dosage should be gradually increased in the patient based on the tolerability of the active ingredient. It is recommended that the dose be doubled after one or two weeks of treatment and - after a further two or three weeks - increased until the target maintenance dose of 10 mg QUARK once daily is reached.
See also the posology described above for patients treated with a diuretic.
Treatment of kidney diseases
In patients with diabetes and microalbuminuria :
Initial dose
The recommended starting dose is 1.25 mg of QUARK once daily.
Titration and maintenance dose
The dosage should be gradually increased in the patient based on the tolerability of the active ingredient.
It is recommended that the single daily dose be doubled to 2.5 mg after two weeks and a further two weeks to 5 mg.
In patients with diabetes and at least one cardiovascular risk factor
Initial dose
The recommended starting dose is 2.5 mg of QUARK once daily.
Titration and maintenance dose
The dosage should be gradually increased in the patient based on the tolerability of the active ingredient.
It is recommended that the single daily dose be doubled to 5 mg of QUARK after one to two weeks and then to 10 mg of QUARK after a further two to three weeks. The target daily dose is 10 mg.
In patients with non-diabetic nephropathy, defined by macroproteinuria ≥3g / day
Initial dose
The recommended starting dose is 1.25 mg of QUARK once daily.
Titration and maintenance dose
The dosage should be gradually increased in the patient based on the tolerability of the active ingredient.
It is recommended that the single daily dose be doubled to 2.5 mg after two weeks and then to 5 mg after a further two weeks.
Symptomatic heart failure
Initial dose
In patients stabilized on diuretic therapy, the recommended starting dose is 1.25 mg per day.
Titration and maintenance dose
QUARK should be titrated by doubling the dose every one to two weeks up to a maximum daily dose of 10 mg. Two administrations per day are preferable.
Secondary prevention in patients with previous acute myocardial infarction and heart failure
Initial dose
After 48 hours of myocardial infarction, in clinically and haemodynamically stable patients, the starting dose is 2.5 mg twice daily for three days. If the starting 2.5 mg dose is not tolerated, a dose should be given. 1.25 mg twice daily for two days before increasing to 2.5 mg and 5 mg twice daily If the dose cannot be increased to 2.5 mg twice daily the treatment should be stopped.
See also the posology described above for patients treated with a diuretic.
Titration and maintenance dose
The daily dose is subsequently increased by doubling it at intervals of one to three days to a maintenance dose of 5 mg twice daily.
Whenever possible, the maintenance dose is divided into two administrations per day.
If the dose cannot be increased to 2.5mg twice daily the treatment should be stopped. There is still insufficient experience in treating patients with severe heart failure (NYHA IV) immediately following myocardial infarction. If a decision is made to treat these patients, it is recommended that therapy be initiated at 1.25 mg once daily and that particular caution be exercised in any dose increase.
Special populations
Patients with impaired renal function
The daily dose in patients with renal insufficiency should be based on creatinine clearance (see section 5.2):
• if creatinine clearance is ≥ 60 ml / min, it is not necessary to adjust the starting dose (2.5 mg / day); the maximum daily dose is 10 mg;
• if the creatinine clearance is between 30-60 ml / min it is not necessary to adjust the starting dose (2.5 mg / day); the maximum daily dose is 5 mg;
• if creatinine clearance is between 10-30 ml / min, the starting dose is 1.25 mg / day and the maximum daily dose is 5 mg;
• Ramipril is poorly dialyzable in hypertensive patients on hemodialysis; the starting dose is 1.25 mg / day and the maximum daily dose is 5 mg; the medicinal product must be administered a few hours after performing dialysis.
Patients with impaired hepatic function (see section 5.2)
In patients with hepatic insufficiency treatment with QUARK should only be initiated under close medical supervision and the maximum daily dose of QUARK is 2.5 mg.
Elderly patients
The starting dose should be the lowest and the subsequent titration should be very gradual due to the increased likelihood of undesirable effects particularly in very elderly or debilitated patients. A reduced starting dose of ramipril of 1.25 mg should be considered.
Pediatric population
The safety and efficacy of ramipril in children has not yet been established. Currently available data for Quark are described in sections 4.8, 5.1, 5.2 and 5.3 but no specific recommendation on a posology can be made.
04.3 Contraindications -
- Hypersensitivity to the active substance, to any of the excipients or to other ACE inhibitors (Angiotensin Converting Enzyme inhibitors) (see section 6.1).
- History of angioedema (hereditary, idiopathic or previous angioedema with ACE inhibitors or AIIRAs).
- Extracorporeal treatments that bring blood into contact with negatively charged surfaces (see section 4.5).
- Significant bilateral stenosis of the renal artery or unilateral stenosis in patients with a single functioning kidney.
- Second and third trimester of pregnancy (see sections 4.4 and 4.6).
- Ramipril should not be used in patients with hypotension or haemodynamically unstable.
- The concomitant use of Quark with aliskiren-containing medicines is contraindicated in patients with diabetes mellitus or renal impairment (glomerular filtration rate GFR
04.4 Special warnings and appropriate precautions for use -
Special populations
• Pregnancy
Therapy with ACE inhibitors, such as ramipril, or with Angiotensin II Receptor Antagonists (AIIRAs) should not be initiated during pregnancy.
For patients planning pregnancy, alternative antihypertensive treatments with a proven safety profile for use in pregnancy should be used unless continued ACE inhibitor / AIIRA therapy is considered essential. When diagnosed with an ACE inhibitor / AIIRA. pregnancy, treatment with ACE inhibitors / AIIRAs should be stopped immediately, and, if appropriate, alternative therapy should be started (see sections 4.3 and 4.6).
• Patients particularly at risk of hypotension
- Patients with overactivation of the renin-angiotensin-aldosterone system
Patients with overactivation of the renin-angiotensin-aldosterone system may experience an acute noticeable drop in blood pressure and deterioration of renal function due to ACE inhibition, especially when the ACE inhibitor or a concomitant diuretic are given for the first time. or at the first dose increase. Relevant activation of the renin-angiotensin-aldosterone system must be expected and medical supervision including blood pressure monitoring is required, for example in:
- patients with severe hypertension;
- patients with decompensated congestive heart failure;
- patients with haemodynamically significant obstacle to left ventricular inflow or outflow (eg aortic or mitral valve stenosis);
- patients with unilateral renal artery stenosis with a functioning second kidney;
- patients in whom fluid or salt depletion exists or may develop (including patients on diuretics);
- patients with liver cirrhosis and / or ascites;
- during major surgery or during anesthesia with drugs that cause hypotension.
It is generally recommended to correct dehydration, hypovolaemia or salt depletion before starting treatment (however in patients with heart failure this corrective action should be carefully weighed against the risk of overload).
- Dual blockade of the renin-angiotensin-aldosterone system (RAAS)
There is evidence that concomitant use of ACE inhibitors, angiotensin II receptor blockers or aliskiren increases the risk of hypotension, hyperkalaemia and decreased renal function (including acute renal failure). Dual blockade of the RAAS through the combined use of ACE inhibitors, angiotensin II receptor blockers or aliskiren is therefore not recommended (see sections 4.5 and 5.1).
If dual block therapy is considered absolutely necessary, this should only be done under the supervision of a specialist and with close and frequent monitoring of kidney function, electrolytes and blood pressure.
ACE inhibitors and angiotensin II receptor antagonists should not be used concomitantly in patients with diabetic nephropathy.
Transient or persistent heart failure after myocardial infarction
• Patients at risk of cardiac or cerebral ischaemia in case of acute hypotension
The initial phase of treatment requires careful medical supervision.
• Elderly patients
See section 4.2.
• Surgery
If possible, it is recommended that treatment with angiotensin converting enzyme inhibitors such as ramipril be discontinued one day before surgery.
• Monitoring of renal function
Renal function should be evaluated before and during treatment and the dose should be adjusted particularly in the first weeks of treatment. Particularly careful monitoring is required in patients with renal insufficiency (see section 4.2). There is a risk of impaired renal function, particularly in patients with congestive heart failure or after a kidney transplant.
• Angioedema
Cases of angioedema have been reported in patients receiving ACE inhibitors including ramipril (see section 4.8).
In case of angioedema, QUARK should be discontinued.
Emergency treatment should be instituted promptly. Patients should be kept under observation for at least 12-24 hours and discharged only after complete resolution of symptoms.
Intestinal angioedema has been reported in patients receiving ACE inhibitors, including QUARK (see section 4.8). These patients presented with abdominal pain (with or without nausea or vomiting).
• Anaphylactic reactions during desensitizing therapies
The likelihood and severity of anaphylactic or anaphylactoid reactions following contact with insect venom or other allergens are increased during therapy with ACE inhibitors. Temporary discontinuation of QUARK should be considered prior to desensitization.
• Hyperkalemia
Hyperkalaemia has been observed in some patients treated with ACE inhibitors including QUARK. Patients at risk for hyperkalaemia include those with renal insufficiency, age> 70 years, with uncontrolled diabetes mellitus or those using potassium salts, potassium-sparing diuretics, or other active substances that increase plasma potassium, or conditions such as dehydration, acute heart failure, metabolic acidosis.
If the use of any of the above substances is deemed necessary, regular monitoring of serum potassium is recommended (see section 4.5).
• Neutropenia / agranulocytosis
Neutropenia / agranulocytosis, as well as thrombocytopenia and anemia, have been observed rarely, and bone marrow depression has also been reported.
It is recommended to monitor the number of white blood cells to allow the detection of a possible leukopenia.
More frequent monitoring is advised in the initial phase of treatment and in patients with impaired renal function, in patients with concomitant collagen disorders (e.g. lupus erythematosus or scleroderma) and in those treated with drugs that can cause changes in the blood picture (see paragraphs 4.5 and 4.8).
• Ethnic differences
ACE inhibitors cause a higher incidence of angioedema in black patients than in non-black patients.
Like other ACE inhibitors, ramipril may be less effective in lowering blood pressure in black populations than in non-black populations, possibly due to a higher prevalence of low-renin hypertension in black populations.
• Cough
Cough has been reported with the use of ACE inhibitors. Typically, cough is nonproductive, persistent and resolves upon discontinuation of therapy. ACE inhibitor cough should be considered in the differential diagnosis of cough.
04.5 Interactions with other medicinal products and other forms of interaction -
Contraindicated associations
Extracorporeal treatments that bring blood into contact with negatively charged surfaces such as dialysis or haemofiltration with high flux membranes (eg polyacrylonitrile membranes) or low density lipoprotein apheresis by means of dextran sulphate are contraindicated due to the increased risk of severe anaphylactoid reactions (see section 4.3). If this type of treatment is required, the use of different dialysis membranes or a different class of antihypertensive agents should be considered.
Clinical trial data have shown that dual blockade of the renin-angiotensin-aldosterone system (RAAS) through the combined use of ACE inhibitors, angiotensin II receptor blockers or aliskiren is associated with a higher frequency of adverse events. such as hypotension, hyperkalaemia and decreased renal function (including acute renal failure) compared to the use of a single agent active on the RAAS system (see sections 4.3, 4.4 and 5.1).
Precautions for use
Potassium salts, heparin, potassium-sparing diuretics and other active substances that increase blood potassium levels (including angiotensin II antagonists, trimethoprim, tacrolimus, cyclosporine):
Hyperkalaemia may occur, therefore careful monitoring of serum potassium levels is required.
Antihypertensive drugs (e.g. diuretics) and other drugs with potential antihypertensive effect (e.g. nitrates, tricyclic antidepressants, anesthetics, alcohol intake, baclofen, alfuzosin, doxazosin, prazosin, tamsulosin, terazosin): A possible potentiation of the risk of hypotension should be anticipated (see section 4.2 for diuretics).
Sympathomimetic vasopressors and other substances (e.g. isoproterenol, dobutamide, dopamide, adrenaline) which can reduce the antihypertensive effect of QUARK: Blood pressure monitoring is recommended.
Allopurinol, immunosuppressants, corticosteroids, procainamide, cytostatics and other drugs that can alter the blood picture: increased risk of haematological reactions (see section 4.4).
Salts of lithium: Lithium excretion can be reduced by ACE inhibitors and therefore lithium toxicity can be increased. Serum lithium levels should be monitored.
Antidiabetic agents including insulin: Hypoglycaemic reactions may occur. Therefore close blood glucose monitoring is recommended.
Non-steroidal anti-inflammatory drugs and acetylsalicylic acid: A possible reduction in the antihypertensive effect of QUARK should be anticipated. In addition, concomitant therapy with ACE inhibitors and NSAIDs may lead to an increased risk of worsening of renal function and an increase in kalaemia.
04.6 Pregnancy and breastfeeding -
Pregnancy
The use of QUARK is not recommended during the first trimester of pregnancy (see section 4.4) and is contraindicated during the second and third trimester of pregnancy (see section 4.3).
Epidemiological evidence on the risk of teratogenicity following exposure to ACE inhibitors during the first trimester of pregnancy has not been conclusive; however a small increase in risk cannot be excluded.
For patients planning pregnancy, alternative antihypertensive treatments with a proven safety profile for use in pregnancy should be used unless continued ACE inhibitor therapy is considered essential.
When pregnancy is diagnosed, treatment with ACE inhibitors should be stopped immediately and, if appropriate, alternative therapy should be started.
Exposure to ACE inhibitors / Angiotensin II Receptor Antagonists (AIIRAs) during the second and third trimesters in women is known to induce fetal toxicity (decreased renal function, oligohydramnios, skull ossification retardation) and neonatal toxicity ( renal failure, hypotension, hyperkalaemia) (see section 5.3 "Preclinical safety data").
Should exposure to ACE inhibitor have occurred from the second trimester of pregnancy, ultrasound check of renal function and skull is recommended.
Neonates whose mothers have taken ACE inhibitors should be carefully observed for hypotension, oliguria and hyperkalaemia (see sections 4.3 and 4.4).
Feeding time
Since there are insufficient data regarding the use of ramipril during lactation (see section 5.2), Quark is not recommended and alternative treatments with a proven safety profile for use during lactation are preferred, especially if breastfeeding. of newborns or premature babies.
04.7 Effects on ability to drive and use machines -
Some undesirable effects (e.g. symptoms of low blood pressure such as dizziness) may interfere with the patient's ability to concentrate and react and therefore represent a risk in situations where these skills are particularly important (e.g. operating machinery or driving vehicles).
This may particularly occur at the start of treatment or when substituting for another therapy. After the first dose or dose increase it is not recommended to drive or operate machinery for several hours.
04.8 Undesirable effects -
The safety profile of ramipril includes persistent dry cough and reactions due to hypotension. Serious adverse reactions include angioedema, hyperkalaemia, hepatic or renal damage, pancreatitis, severe skin reactions and neutropenia / agranulocytosis.
The frequency of undesirable effects is defined using the following convention:
Very common (≥ 1/10); common (≥ 1/100,
Within the frequency groups, undesirable effects are listed in descending order of severity.
Pediatric population
The safety of ramipril was monitored in 325 children and adolescents, aged 2-16 years in 2 clinical studies. Although the nature and severity of adverse events are similar to those in adults, the frequency of the following events is higher in children:
- Tachycardia, nasal congestion and rhinitis, "common" (i.e. ≥ 1/100,
- "Common" conjunctivitis (i.e. ≥ 1/100,
- Tremor and "uncommon" urticaria (ie ≥ 1 / 1,000. The overall safety profile of ramipril in pediatric patients does not differ significantly from the safety profile in adults.
Reporting of suspected adverse reactions.
Reporting of suspected adverse reactions occurring after authorization of the medicinal product is important as it allows continuous monitoring of the benefit / risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system. "address www.agenziafarmaco.gov.it/it/responsabili
04.9 Overdose -
Symptoms associated with ACE inhibitor overdose may include excessive peripheral vasodilation (with marked hypotension, shock), bradycardia, electrolyte disturbance, renal failure. Patients should be closely monitored and treatment should be symptomatic and supportive. The main measures suggested include detoxification (gastric lavage, administration of adsorbents) and measures to restore haemodynamic stability, including administration of alpha 1 adrenal agonists or angiotensin II (angiotensinamide). Ramiprilat, the active metabolite of ramipril is poorly removed from the general circulation by hemodialysis.
05.0 PHARMACOLOGICAL PROPERTIES -
05.1 "Pharmacodynamic properties -
Pharmacotherapeutic group: ACE inhibitors.
A.T.C Code: C09AA05.
Mechanism of action
Ramiprilat, the active metabolite of the prodrug ramipril, inhibits the dipeptidylcarboxypeptidase I enzyme (synonyms: angiotensin converting enzyme; kininase II). This enzyme, at plasma and tissue level, determines the conversion of angiotensin I into the vasoconstrictor substance angiotensin II , and the degradation of the vasodilator bradykinin. The reduced formation of angiotensin II and the inhibition of the degradation of bradykinin lead to vasodilation.
Since angiotensin II also stimulates the release of aldosterone, ramiprilat causes a reduction in the secretion of aldosterone.
The mean response to ACE inhibitors of black (Afro-Caribbean) hypertensive patients (usually this hypertensive population has a low renin level) is lower than that of non-black patients.
Pharmacodynamic effects
Antihypertensive properties:
Administration of ramipril causes a marked reduction in peripheral arterial resistance. Generally neither the renal plasma flow nor the glomerular filtration index undergo notable changes.
Administration of ramipril to hypertensive patients results in a reduction in blood pressure in both standing and supine positions, without compensatory increase in heart rate.
After a single oral dose, in most patients the antihypertensive action occurs 1-2 hours after intake, reaches its maximum effect after 3-6 hours and lasts for at least 24 hours.
The maximum antihypertensive effect of continuous treatment with ramipril is generally achieved after 3-4 weeks of treatment.
It has been shown that the antihypertensive effect is maintained for prolonged therapy up to 2 years.
Abrupt discontinuation of therapy does not cause a rapid rebound increase in blood pressure.
Heart failure:
Ramipril has been shown to be effective, in addition to conventional therapy with diuretics and cardiac glucosides, in patients with functional classes II-IV defined by the New-York Heart Association. The drug had beneficial effects on cardiac hemodynamics (decreased filling pressure of the left and right ventricles, decreased total peripheral vascular resistance, increased cardiac output, and improved cardiac index).It also reduces neuroendocrine activation.
Clinical efficacy and safety
Cardiovascular prevention / nephroprotection:
A placebo-controlled prevention study (the HOPE study) was conducted in which ramipril was added to standard therapy in more than 9,200 patients. Patients with an increased risk of cardiovascular disease resulting from atherothrombotic cardiovascular disease (coronary artery disease, stroke or peripheral vascular disease) or diabetes mellitus with at least one additional risk factor (documented microalbuminuria, hypertension, high total cholesterol level, low HDL cholesterol level, or smoking), were included in the study.
The study showed that ramipril statistically significantly decreases the incidence of myocardial infarction, cardiovascular death and stroke, alone or combined (primary events combined).
HOPE study: main results
Ramipril Placebo relative risk (95% confidence interval) p-value % % All patients n = 4.645 n = 4.652 Combined primary event 14.0 17.8 0.78 (0.70 - 0.86) Myocardial infarction 9.9 12.3 0.80 (0.70-0.90) Death from cardiovascular causes 6.1 8.1 0.74 (0.64-0.87) Stroke 3.4 4.9 0.68 (0.56-0.84) Secondary endpoints Death for any cause 10.4 12.2 0.84 (0.75-0.95) 0.005 Need for revascularization 16.0 18.3 0.85 (0.77-0.94) 0.002 Hospitalization for unstable angina 12.1 12.3 0.98 (0.87-1.10) NS Hospitalization for heart failure 3.2 3.5 0.88 (0.70-1.10) 0.25 Complications related to diabetes 6.4 7.6 0.84 (0.72-0.98) 0.03 The MICRO-HOPE study, a predefined substudy from the HOPE study, evaluated the effect of adding ramipril 10 mg to the current regimen versus placebo in 3,577 patients aged ≥ 55 years (with no upper age limit), the majority with type 2 diabetes (and at least one other CV risk factor) normotensive or hypertensive.
The primary analysis of the results showed that 117 (6.5%) participants treated with ramipril and 149 (8.4%) treated with placebo developed overt nephropathy, which corresponds to a Relative Risk Reduction (RRR) of 24%. ; 95% CI [3-40], p = 0.027.
The REIN randomized, double-blind, parallel-group, placebo-controlled study was aimed at demonstrating the effect of ramipril treatment on the rate of decrease in glomerular function (GFR) in 352 normotensive or hypertensive patients (18-70 years). of age) with mild proteinuria (i.e. urinary protein excretion> 1 e
The main analysis of patients with the most severe proteinuria (layer prematurely separated due to the benefit seen in the ramipril group) showed that the mean rate of decrease in GFR per month was lower with ramipril than with placebo; -0, 54 vs. -0.88 mL / min / month, p = 0.038. The difference between groups was 0.34 [0.03-0.65] per month, and approximately 4 mL / min / year; on 23, 1% of patients in the ramipril group achieved the combined secondary endpoint of doubling baseline serum creatinine concentration and / or end-stage renal failure (ESRD) (need for dialysis or kidney transplantation) versus 45.5% in the group placebo (p = 0.02).
Secondary prevention after acute myocardial infarction
The AIRE study included more than 2,000 patients with transient / persistent clinical signs of heart failure after documented myocardial infarction. Ramipril treatment started 3-10 days after acute myocardial infarction. The study indicated that after a mean follow-up time of 15 months the mortality in ramipril-treated patients was 16.9% while in patients treated with ramipril. treated with placebo was 22.6%, which means an absolute reduction in mortality of 5.7% and a relative risk reduction of 27% (CI of 95% [11- 40%]).
Two large randomized controlled trials (ONTARGET (ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial) and VA Nephron-D (The Veterans Affairs Nephropathy in Diabetes)) have examined the use of the combination of an ACE inhibitor with an antagonist of the angiotensin II receptor.
ONTARGET was a study conducted in patients with a history of cardiovascular or cerebrovascular disease, or type 2 diabetes mellitus associated with evidence of organ damage. VA NEPHRON-D was a study conducted in patients with type 2 diabetes mellitus and diabetic nephropathy.
These studies did not demonstrate any significant beneficial effect on renal and / or cardiovascular outcomes and mortality, while an increased risk of hyperkalaemia, acute renal injury and / or hypotension was observed compared to monotherapy.
These results are also relevant for other ACE inhibitors and angiotensin II receptor antagonists, given their similar pharmacodynamic properties.
ACE inhibitors and angiotensin II receptor antagonists should therefore not be used simultaneously in patients with diabetic nephropathy.
ALTITUDE (Aliskiren Trial in Type 2 Diabetes Using Cardiovascular and Renal Disease Endpoints) was a study aimed at verifying the advantage of adding aliskiren to standard therapy of an ACE inhibitor or angiotensin II receptor antagonist in patients with diabetes mellitus. type 2 and chronic kidney disease, cardiovascular disease, or both. The study was terminated early due to an increased risk of adverse events. Cardiovascular death and stroke were both numerically more frequent in the aliskiren group than in the placebo group, and adverse events and serious adverse events of interest (hyperkalaemia, hypotension and renal dysfunction) were reported more frequently in the aliskiren group than in the placebo group.
Pediatric population
In a randomized, double-blind clinical trial involving 244 pediatric hypertension patients (73% primary hypertension), aged 6-16 years, patients received low-dose, medium-dose, or high-dose ramipril for achieve plasma concentrations of ramiprilat corresponding to adult dosages of 1.25 mg, 5 mg and 20 mg based on body weight. At the end of 4 weeks, ramipril was ineffective in meeting the endpoint of lowering systolic blood pressure but at the higher dose it reduced diastolic blood pressure. Both medium and high doses of ramipril showed significant reduction of both systolic and diastolic blood pressure in children with confirmed hypertension.
This effect was not observed in a randomized, double-blind, 4-week clinical study in which
the dose was progressively increased, conducted on 218 pediatric patients aged 6-16 years (75% primary hypertension), in whom both diastolic and systolic blood pressure showed a modest rebound effect, but not a statistically significant return to baseline, in all three ramipril dose levels assessed by weight, low dose (0.625 mg - 2.5 mg), medium dose (2.5 mg - 10 mg) or high dose (5 mg - 20 mg) mg). Ramipril did not have a linear dose-dependent response in the pediatric population studied.
05.2 "Pharmacokinetic properties -
Pharmacokinetics and Metabolism
Absorption
After oral administration, ramipril is rapidly absorbed from the gastrointestinal tract: the peak plasma concentration of ramipril is reached within one hour. Based on urinary recovery, absorption is at least 56% and is not significantly affected by the presence. of food in the gastrointestinal tract. The bioavailability of the active metabolite ramiprilat after oral administration of 2.5 mg and 5 mg of ramipril is 45%.
Peak plasma concentrations of ramiprilat, the only active metabolite of ramipril, are reached 2-4 hours after ramipril intake. Steady-state plasma concentrations of ramiprilat after once daily administration of the usual daily doses of ramipril are reached by the fourth day of treatment approx.
Distribution
The serum protein binding of ramipril is approximately 73% and that of ramiprilat is approximately 56%.
Metabolism
Ramipril is almost completely metabolised to ramiprilat and the diketopiperazine ester, the acid form of diketopiperazine and the glucuronides of ramipril and ramiprilat.
Elimination
The excretion of the metabolites is mainly via the kidney.
Plasma concentrations of ramiprilat decrease in a polyphasic manner. Due to its potent and saturable binding to ACE and slow dissociation from the enzyme, ramiprilat exhibits a prolonged terminal elimination phase at very low plasma concentrations.
After multiple daily doses of ramipril, the effective half-life of ramiprilat concentrations was 13-17 hours for the 5-10 mg doses and longer for the lower 1.25-2.5 mg doses. This difference is related to the saturable ability of the enzyme to bind ramiprilat.
Feeding time
A single oral dose of ramipril produced an undetectable level of ramipril and its metabolite in breast milk. However, the effects of multiple doses are not known.
Patients with renal insufficiency (see section 4.2)
The renal excretion of ramiprilat is reduced in patients with renal insufficiency and the renal clearance of ramiprilat is proportional to the creatinine clearance. This results in elevated plasma concentrations of ramiprilat which decline more slowly than in patients with normal renal function.
Patients with hepatic insufficiency (see section 4.2)
In patients with impaired hepatic function, the metabolization of ramipril to ramiprilat is delayed due to decreased activity of hepatic esterases; in these patients the plasma levels of ramipril are increased. Peak concentrations of ramiprilat in these patients, however, they are not different from those seen in subjects with normal liver function.
Pediatric population
The pharmacokinetic profile of ramipril was studied in 30 hypertensive pediatric patients aged 2-16 years, weighing ≥ 10 kg. After administration of doses of 0.05 to 0.2 mg / kg ramipril was rapidly and largely metabolised to ramiprilat. Peak plasma concentrations of ramiprilat occur within 2-3 hours. Ramiprilat clearance is highly correlated with the log of body weight (p
The dose of 0.05 mg / kg in children achieved exposure levels similar to those seen in adults treated with 5 mg of ramipril. The dose of 0.2 mg / kg in children resulted in higher exposure levels than the maximum recommended dose of 10 mg per day for adults.
05.3 Preclinical safety data -
Oral administration of ramipril was found to be devoid of acute toxicity in rodents and dogs. Studies involving chronic oral administration were conducted in rats, dogs and monkeys. Alterations of plasma electrolytes were detected in the three species. As an expression of the pharmacodynamic activity of ramipril, a pronounced enlargement of the juxtaglomerular apparatus was found in dogs and monkeys starting with daily doses of 250 mg / kg. Rats, dogs and monkeys tolerated daily doses of 2, 2.5 and 8 mg / kg respectively without adverse effects.
Irreversible renal damage was observed in very young rats treated with a single dose of ramipril.
Reproductive toxicology studies in rats, rabbits and monkeys revealed no teratogenic properties. Fertility was not affected in male or female rats.
Administration of ramipril to female rats during the gestation and lactation period resulted in irreversible renal damage (dilation of the renal pelvis) in the offspring at daily doses of 50 mg / kg body weight or higher.
The mutagenicity test, conducted using various test systems, did not provide evidence that ramipril possesses mutagenic or genotoxic properties.
06.0 PHARMACEUTICAL INFORMATION -
06.1 Excipients -
Quark 2.5 mg tablets
hypromellose, pregelatinised maize starch, microcrystalline cellulose, sodium stearyl fumarate, yellow iron oxide E 172.
Quark 5 mg tablets
hypromellose, pregelatinised maize starch, microcrystalline cellulose, sodium stearyl fumarate, red iron oxide E 172.
Quark 10 mg tablets
hypromellose, pregelatinised maize starch, microcrystalline cellulose, sodium stearyl fumarate.
06.2 Incompatibility "-
Not relevant.
06.3 Period of validity "-
5 years.
06.4 Special precautions for storage -
They are not provided.
06.5 Nature of the immediate packaging and contents of the package -
Blister packs in opaque white PVC and aluminum, heat-sealed.
Quark 2.5 mg tablets, 28 divisible tablets
Quark 5 mg tablets, 14 divisible tablets
Quark 10 mg tablets, 28 divisible tablets
06.6 Instructions for use and handling -
No special instructions.
Unused medicine and waste derived from this medicine must be disposed of in accordance with local regulations.
07.0 HOLDER OF THE "MARKETING AUTHORIZATION" -
POLIFARMA S.p.A. Viale dell "Arte, 69 - 00144 ROME
08.0 MARKETING AUTHORIZATION NUMBER -
Quark 2.5 mg tablets - 28 tablets A.I.C. n .: 027162054
Quark 5 mg tablets - 14 tablets A.I.C. n .: 027162066
Quark 10 mg tablets - 28 tablets A.I.C. n .: 027162078
09.0 DATE OF FIRST AUTHORIZATION OR RENEWAL OF THE AUTHORIZATION -
First Authorization Date: March 1990 Quark 2.5 mg, 5 mg.
April 2004 Quark 10 mg
Last renewal date: June 2010
10.0 DATE OF REVISION OF THE TEXT -
December 2014
11.0 FOR RADIO DRUGS, COMPLETE DATA ON THE INTERNAL RADIATION DOSIMETRY -
12.0 FOR RADIO DRUGS, FURTHER DETAILED INSTRUCTIONS ON EXEMPORARY PREPARATION AND QUALITY CONTROL -