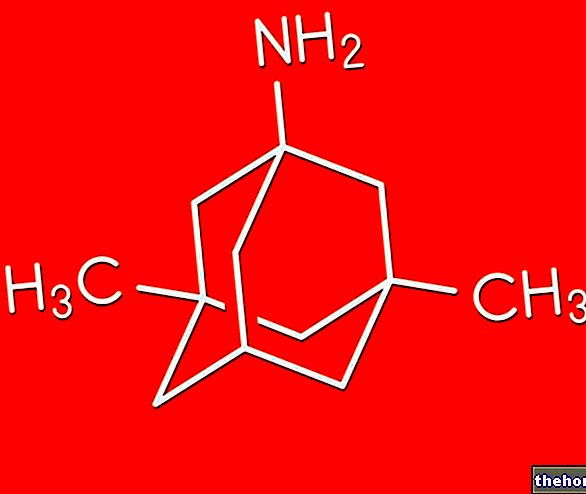
Active ingredients: Clarithromycin
Veclam 125 mg / 5 ml granules for oral suspension
Veclam package inserts are available for pack sizes:- Veclam 125 mg / 5 ml granules for oral suspension
- Veclam 250 mg / 5 ml granules for oral suspension
- Veclam 250 mg coated tablets
- Veclam 500 mg coated tablets
- Veclam RM 500 mg modified release tablets
- Veclam 500 mg granules for oral suspension
- Veclam 500 mg / 10 ml powder and solvent for solution for infusion
Why is Veclam used? What is it for?
PHARMACOTHERAPEUTIC CATEGORY
General antibacterial for systemic use - Macrolides
THERAPEUTIC INDICATIONS
Treatment of infections caused by pathogens sensitive to clarithromycin. Infections of the rhino-pharyngeal tract (tonsillitis, pharyngitis), of the paranasal sinuses. Acute Otitis Media (AOM). Lower respiratory tract infections: bronchitis, bacterial pneumonia and atypical pneumonia. Skin infections: impetigo, erysipelas, folliculitis, furunculosis and infected wounds.
Veclam 125 mg / 5 ml granules for oral suspension is indicated in children from 6 months to 12 years of age.
Contraindications When Veclam should not be used
Hypersensitivity to macrolide class antibiotics or to any of the excipients listed in the "Composition" section.
Concomitant administration of clarithromycin with any of the following drugs is contraindicated: astemizole, cisapride, pimozide, terfenadine as they can induce QT interval prolongation and cardiac arrhythmia, including ventricular tachycardia, ventricular fibrillation and torsades de pointes.
Concomitant administration of clarithromycin with ticagrelor or ranolazine is contraindicated.
Concomitant administration of clarithromycin and ergot alkaloids (ergotamine or dihydroergotamine) which may lead to ergot toxicity is contraindicated (see section "Interactions").
The concomitant administration of clarithromycin and midazolam for oral use is contraindicated (see section "Interactions").
Veclam should not be used in patients with congenital or acquired QT interval prolongation documented and with a history of ventricular arrhythmia (see section "Special warnings").
Veclam must not be administered concomitantly with HMG-CoA reductase inhibitors (statins), which are extensively metabolised by CYP3A4 (lovastatin and simvastatin), due to the increased risk of myopathy, including rhabdomyolysis (see section "Interactions").
Veclam must not be given to patients with hypokalaemia (risk of QT interval prolongation).
Veclam must not be used in patients suffering from severe hepatic insufficiency associated with kidney damage.
As with other potent inhibitors of the CYP3A4 enzyme, clarithromycin should not be used concomitantly with colchicine (see section "Special warnings").
Precautions for use What you need to know before taking Veclam
As clarithromycin is metabolized and excreted mainly in the liver, particular caution should be exercised when administering the drug to patients with impaired hepatic function, and in subjects with moderate or severe renal impairment.
Fatal cases of liver failure have been reported with the use of clarithromycin. Some patients may have had previous liver disease or taken other hepatotoxic medicinal products.
The patient should be advised to discontinue treatment and contact their physician if signs and symptoms of liver disease such as anorexia, jaundice, dark urine, itching or abdominal pain occur.
Cases of pseudomembranous colitis, ranging in severity from moderate to life-threatening, have been reported with the use of nearly all antibacterials, including macrolides. Clostridium difficile diarrhea (CDAD) has been reported. with the use of most antibacterials, including clarithromycin, which can range from moderate diarrhea to fatal colitis. Antibacterial treatment alters normal intestinal flora, which can lead to excessive proliferation of C. difficile.
In all patients who complain of diarrhea after taking antibiotics, the presence of CDAD should be evaluated. These patients should undergo a careful medical history as it has been reported that CDAD may occur during the two months following the intake of antibacterials. Therefore, discontinuation of clarithromycin treatment should take place regardless of the therapeutic indication. A microbial test should be performed and appropriate treatment initiated. The administration of antiperistaltic agents should be avoided.
Interactions Which drugs or foods can change the effect of Veclam
Tell your doctor or pharmacist if you have recently taken any other medicines, even those without a prescription.
The use of the following medicines is absolutely contraindicated due to the potential serious effects due to their drug interaction: astemizole, cisapride, pimozide, terfenadine.
Concomitant intake resulted in prolonged QT interval, cardiac arrhythmias including ventricular tachycardia, ventricular fibrillation and torsades de pointes (see section "Contraindications").
Some post-marketing reports indicate that co-administration of clarithromycin and ergotamine or dihydroergotamine has been associated with acute ergot toxicity (ergotism) characterized by vasospasm and ischaemia of the extremities and other tissues, including the central nervous system. The concomitant administration of clarithromycin and ergot alkaloids is contraindicated (see section "Contraindications").
Concomitant use of clarithromycin and lovastatin or simvastatin is contraindicated as these statins are extensively metabolised by CYP3A4 and concomitant treatment with clarithromycin increases their plasma concentration, which increases the risk of myopathy, including rhabdomyolysis (see section "Contraindications" ).
There have been reports of rhabdomyolysis in patients taking clarithromycin concomitantly with these statins. If treatment with clarithromycin cannot be avoided, therapy with lovastatin or simvastatin should be discontinued during treatment.
Care should be taken when prescribing clarithromycin with statins. In situations where concomitant use of clarithromycin and statins cannot be avoided, it is recommended to prescribe the lowest registered dose of statins. The use of a statin that is not dependent on CYP3A metabolism (eg. fluvastatin). Patients should be monitored for signs and symptoms of myopathy.
Effects of other medicinal products on clarithromycin:
Drugs that induce CYP3A (e.g. rifampicin, phenytoin, carbamazepine, phenobarbital, St. John's wort) may induce the metabolism of clarithromycin. This leads to sub-therapeutic levels of clarithromycin with reduced therapeutic efficacy. Drugs that are strong inducers of cytochrome P450 metabolism such as efavirenz, nevirapine, rifampicin, rifabutin and rifapentine can accelerate the metabolism of clarithromycin and consequently lower the plasma levels of clarithromycin, while increasing the plasma levels of 14-OH-clarithromycin, a metabolite that is also active from a microbiological point of view.
A pharmacokinetic study has shown that the concomitant administration of 200 mg of ritonavir every 8 hours and 500 mg of clarithromycin every 12 hours leads to marked inhibition of the metabolism of clarithromycin. Complete inhibition of 14-OH clarithromycin formation was noted.
Clarithromycin exposure was reduced by etravirine; however, the concentration of the active metabolite, 14-OH-clarithromycin, was increased. Since 14-OH-clarithromycin has reduced activity against Mycobacterium Avium Complex (MAC), overall activity against this pathogen may be altered, therefore alternatives to clarithromycin should be considered for the treatment of MAC.
Concomitant administration of fluconazole 200 mg daily and clarithromycin 500 mg twice daily to 21 healthy volunteers resulted in increases in the mean minimum clarithromycin concentration (Cmin) and area under the curve (AUC). ) of 33% and 18%, respectively.Basal concentrations of the active metabolite, 14-OH-clarithromycin, were not significantly affected by concomitant administration of fluconazole.
No dosage adjustment is required for clarithromycin.
Effects of clarithromycin on other medicinal products:
Concomitant administration of clarithromycin, which is known to inhibit CYP3A, and a drug metabolised primarily by CYP3A, may be associated with increases in drug concentrations which may potentiate or prolong the therapeutic and adverse effects of the drug administered in concomitance.
Clarithromycin should be used with caution in patients receiving other drugs that are thought to be substrates of the CYP3A enzyme, especially if the CYP3A substrate has a narrow margin of safety (e.g. carbamazepine) and / or if the substrate is metabolised. extensively by this enzyme.
Dosage adjustments should be considered and, whenever possible, serum concentrations of drugs metabolised primarily by CYP3A should be carefully monitored in patients receiving concomitant clarithromycin therapy.
Drugs or drug classes known or believed to be metabolised by the same CYP3A isozyme are: alprazolam, oral anticoagulants (e.g. warfarin), astemizole, carbamazepine, cilostazol, cisapride, cyclosporine, disopyramide, ergot alkaloids, lovastamolone, methylprednis omeprazole, pimozide, quinidine, rifabutin, sildenafil, simvastatin, sirolimus, tacrolimus, terfenadine, triazolam and vinblastine, but this list is not complete.
Other drugs interacting with a similar mechanism within the cytochrome P450 system are phenytoin, theophylline and valproate. Cases of increased serum levels have been reported. Other cases of torsades de pointes have been reported following concomitant use of clarithromycin and quinidine or disopyramide. Monitor serum concentrations of these drugs while using clarithromycin therapy.
Cases of hypoglycaemia have been reported following the concomitant use of clarithromycin and disopyramide. Monitor blood glucose levels during therapy. In the case of concomitant use of clarithromycin with certain hypoglycemic drugs such as nateglinide and repaglinide, inhibition of the enzyme CYP3A by clarithromycin can occur and can cause hypoglycaemia.Careful monitoring of glucose levels is recommended.
Omeprazole
Healthy adult subjects received clarithromycin (500 milligrams every 8 hours) in combination with omeprazole (40 milligrams daily). Baseline plasma concentrations of omeprazole were increased (Cmax, AUC0-24, and T1 / 2 are increased by 30%, 89% and 34% respectively) due to concomitant administration of clarithromycin.
The mean gastric pH value over 24 hours was 5.2 when omeprazole was administered alone, and was 5.7 when omeprazole was administered concomitantly with clarithromycin.
Sildenafil, tadalafil and vardenafil
Each of these phosphodiesterase inhibitors is metabolised, at least partially, by CYP3A and CYP3A may be inhibited by concomitant administration of clarithromycin. Concomitant administration of clarithromycin and sildenafil, tadalafil or vardenafil is very likely to result in increased exposure to the phosphodiesterase inhibitor. Therefore, a reduction in the dosage of sildenafil, tadalafil and vardenafil should be considered when these drugs are co-administered with clarithromycin.
Results of clinical studies have shown that plasma levels of carbamazepine and theophylline may undergo a modest but statistically significant increase when these are co-administered with clarithromycin.
Tolterodina
The major metabolic pathway of tolterodine passes through the 2D6 isoform of cytochrome P450 (CYP2D6). However, in a population subset without CYP2D6, the identified metabolic pathway is CYP3A. In this population subset, CYP3A inhibition. results in significantly higher serum concentrations of tolterodine. In the presence of CYP3A inhibitors, a dose reduction of tolterodine may be necessary as well as a dose reduction of clarithromycin in the patient population in whom CYP2D6 is poorly metabolised.
Other drug interactions:
Caution is recommended in concomitant administration of clarithromycin and other ototoxic drugs, in particular aminoglycosides (see section "Warnings").
Colchicine is a substrate of both CYP3A and the efflux transporter P-glycoprotein (Pgp). Clarithromycin and other macrolides are known to inhibit CYP3A and Pgp. When clarithromycin and colchicine are administered simultaneously, CYP3A inhibition. and / or Pgp by clarithromycin may lead to increased exposure to colchicine. Monitor patients for clinical symptoms of colchicine toxicity (see "Precautions for use" section).
Patients treated with clarithromycin and digoxin have shown increased serum concentrations of the latter; therefore digoxin levels should be monitored. of zidovudine at steady state.
Since clarithromycin appears to interfere with the absorption of concomitantly administered orally administered zidovudine, this interaction can be strongly avoided by increasing the doses of clarithromycin and zidovudine to allow an interval of at least 4 hours.
This interaction does not appear in pediatric patients with HIV infections when clarithromycin is taken in the granular form at the same time as zidovudine or didanosine.
Phenytoin and valproate:
There have been spontaneous or published reports of interactions of CYP3A inhibitors, including clarithromycin, with drugs not considered to be metabolised by CYP3A (e.g. phenytoin and valproate). Serum level determinations are recommended for these drugs when administered concomitantly with clarithromycin. Cases of elevated serum levels have been reported.
Bidirectional drug interactions:
Clarithromycin and atazanavir, like itraconazole and saquinavir, are substrates and inhibitors of CYP3A and there is evidence of bidirectional drug interactions between these drugs.
Caution is advised in concomitant administration of clarithromycin and calcium channel blockers metabolised by CYP3A4 (e.g. verapamil, amlodipine, diltiazem) due to the risk of hypotension. Plasma concentrations of clarithromycin as well as those of calcium channel blockers may increase due to the interaction. Hypotension, bradyarrhythmia and lactic acidosis have been observed in patients taking clarithromycin and verapamil concomitantly.
Warnings It is important to know that:
Caution should be exercised in those patients with severe renal insufficiency (see section "Dose, method and time of administration"). Since clarithromycin is mainly excreted in the liver, particular caution should be exercised when administering the antibiotic to patients with impaired hepatic function and in subjects with moderate or severe renal impairment. Fatal cases of hepatic failure have been reported.
Plasma levels of clarithromycin do not appear to be appreciably changed by hemodialysis or peritoneal dialysis.
The use of most antibacterials, including macrolides, may result in pseudomembranous colitis and mild to very severe Clostridium difficile diarrhea. Post-marketing cases of colchicine toxicity have been reported with concomitant use. of colchicine and clarithromycin, especially in elderly patients, some of the reported cases have occurred in patients with renal insufficiency and deaths have been reported in some of these patients (see section "Interactions").
Concomitant administration of clarithromycin and colchicine is contraindicated (see section "Contraindications").
Caution is recommended in concomitant administration of clarithromycin and triazolobenzodiazepines, such as triazolam and injectable midazolam (see section "Interactions"). Caution is recommended in concomitant administration of clarithromycin and other ototoxic drugs, especially aminoglycosides. It is therefore advisable to periodically monitor vestibular and auditory function during and after treatment.
Due to the risk of QT interval prolongation, clarithromycin should be used with caution in patients with coronary artery disease, severe heart failure, hypomagnesaemia, bradycardia (
In anticipation of the emerging resistance of Streptococcus pneumoniae to macrolides, it is important to perform a susceptibility test before prescribing clarithromycin for the treatment of community-acquired pneumonia. In hospital-acquired pneumonia, clarithromycin should be administered in combination with appropriate additional antibiotics.
Medium to moderate skin and soft tissue infections are most often caused by Staphylococcus aureus and Streptococcus pyogenes, both of which may be resistant to macrolides. Then it is necessary to carry out a sensitivity test. In cases where beta-lactam antibiotics cannot be used (e.g. allergies), it is preferable to use other antibiotics, such as clindamycin.
In the event of severe acute hypersensitivity reactions such as anaphylaxis, Stevens-Johnson syndrome, toxic epidermal necrolysis and DRESS syndrome, clarithromycin therapy should be discontinued immediately and appropriate treatment adopted immediately.
Concomitant use of clarithromycin and lovastatin or simvastatin is contraindicated (see section "Contraindications"). Care should be taken when prescribing clarithromycin with other statins. Rhabdomyolysis has been reported in patients taking clarithromycin and statins. Patients should be monitored for Signs and Symptoms of Myopathy In situations where concomitant use of clarithromycin and statins cannot be avoided, it is recommended to prescribe the lowest registered dose of statins.The possibility of using a statin that is not dependent on the metabolism of the CYP3A enzyme (eg fluvastatin) may be considered (see section "Interactions").
"Concomitant use of clarithromycin and oral hypoglycemic agents (such as sulfonylureas) and / or insulin may lead to severe hypoglycaemia. C" is the risk of severe bleeding and a significant increase in the international normalized ratio (INR) and prothrombin time when clarithromycin is co-administered with warfarin (see section "Interactions").
Prolonged use of the drug, similarly to what happens with other antibiotics, can cause superinfections from resistant bacteria or fungi. Should superinfections develop, treatment should be discontinued and appropriate therapy initiated immediately. Attention should be paid to the possibility of cross-resistance between clarithromycin and other macrolides, lincomycin and clindamycin.
Important information about some of the excipients
Veclam Granules for oral suspension contains sucrose. Patients diagnosed by their doctor with "intolerance to some sugars should contact him before taking this medicine."
When prescribing Veclam granules for oral suspension to diabetic patients, the sucrose content should be considered.
The medicine is not contraindicated for people with celiac disease.
Veclam Granules for oral suspension also contains castor oil, which can cause stomach upset and diarrhea.
Pregnancy and breastfeeding
Ask your doctor or pharmacist for advice before taking any medicine. Clarithromycin should not be prescribed to pregnant women without a "careful benefit / risk assessment, particularly during the first trimester of pregnancy (see section" Special warnings ").
Clarithromycin is excreted in breast milk in such quantities that effects on the newborns / infants are likely.
Effects on ability to drive and use machines
There are no data on the effect of clarithromycin on the ability to drive or use machines. The risk of vertigo, dizziness, confusion and disorientation, which may occur following administration, must be considered before the patient drives or operates machinery.
Dosage and method of use How to use Veclam: Dosage
Children aged 6 months to 12 years
The recommended daily dosage of clarithromycin in children aged 6 months to 12 years is 7.5 mg / kg given twice daily for non-mycobacterial infections. The usual duration of treatment is 5-10 days depending on the pathogens involved and the severity of the situation.
Dosage scheme Veclam 125 mg / 5 ml granules for oral suspension Use of the spoon:
Preparation of the suspension: To prepare the suspension of Veclam add water to the granules contained in the bottle up to the red line on the bottle.
Shake well. Add more water to bring it back to the line.
The suspension thus prepared has a concentration equal to 2.5% and can be stored at room temperature for 14 days.
Shake well before each use.
In patients with renal insufficiency with a creatinine clearance value of less than 30 ml / min, the dosage of clarithromycin should be halved. In these patients, treatment should not be continued for more than 14 days.
Overdose What to do if you have taken too much Veclam
In case of accidental ingestion / intake of an excessive dose of Veclam, notify your doctor immediately or go to the nearest hospital.
Gastrointestinal disturbances may occur when high doses of clarithromycin are taken. Adverse reactions that occur in the event of overdose should be treated with immediate elimination of the unabsorbed drug and with appropriate supportive therapies. As with other macrolides, serum levels of clarithromycin are not eliminated by hemodialysis or peritoneal dialysis, therefore it is necessary to intervene as soon as possible trying to eliminate the drug not yet absorbed by acting simultaneously with appropriate symptomatic therapy.
If you have any questions about the use of Veclam, ask your doctor or pharmacist.
Side Effects What are the side effects of Veclam
Like all medicines, Veclam can cause side effects, although not everybody gets them. The reported side effects for Veclam are listed below.
to. Summary of the safety profile
The most frequent and common adverse reactions related to clarithromycin therapy for both adult and pediatric patients are abdominal pain, diarrhea, nausea, vomiting and perversion of taste. These adverse events are usually of medium intensity and are consistent with the known safety profile for macrolide antibiotics.
There is no significant difference in the incidence of these gastrointestinal adverse reactions during clinical trials between patients with or without pre-existing mycobacterial infections.
b. Summary table of adverse reactions
The following table summarizes the adverse reactions reported during clinical trials and post-marketing experience with clarithromycin immediate release tablets, granules for oral suspension and modified release tablets.
Adverse reactions considered possibly related to clarithromycin are reported by organ type and frequency, according to the following convention: very common (≥1 / 10), common (≥1 / 100,
* Since these reactions have been reported voluntarily from a population of an indefinite size, it is not always possible to make a true estimate of the frequency or establish a cause-and-effect relationship with drug exposure. patient exposure exceeds one billion days of patient treatment with clarithromycin
** In some of the reported cases of rhabdomyolysis, clarithromycin was administered concomitantly with statins, fibrates, colchicine or allopurinol.
1 Adverse reaction reported for powder and solvent formulation for solution for infusion only
2 Adverse reaction reported for granules for oral suspension only
3 Adverse reaction reported for immediate release tablet formulation only
4, 6, 8,9 See paragraph a)
5, 7, 10 See paragraph c)
c. Description of selected adverse reactions
In some of the reported cases of rhabdomyolysis, clarithromycin was administered concomitantly with statins, fibrates, colchicine or allopurinol.
Post-marketing cases of drug interactions and central nervous system (CNS) effects (eg somnolence and confusion) have been reported with the concomitant use of clarithromycin and triazolam. It is suggested that the patient be monitored for increased pharmacological effects at CNS level.
d. Pediatric populations
Clinical studies have been conducted by administering the clarithromycin-based pediatric suspension to children from 6 months to 12 years of age. Consequently, children under 12 years of age should take the pediatric suspension.
The frequency, type and severity of adverse reactions are expected to be comparable to those occurring in adults.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. Undesirable effects can also be reported directly via the national reporting system at "www.agenziafarmaco.gov.it/it/responsabili". By reporting side effects you can help provide more information on the safety of this medicine. "
Expiry and Retention
Expiry: See the expiry date printed on the package.
The expiry date indicated refers to the product in intact packaging, correctly stored.
This medicine does not require any special storage conditions.
WARNING: Do not use the medicine after the expiry date indicated on the package.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines you no longer use. This will help protect the environment.
Keep this medicine out of the sight and reach of children
Composition and pharmaceutical form
COMPOSITION:
100 ml of reconstituted suspension contains:
Active ingredient: clarithromycin 2.5 g
Excipients: Carbopol 974, povidone, hypromellose phthalate, castor oil, silica gel, sucrose, xanthan gum, mixed fruit flavor, potassium sorbate, citric acid, titanium dioxide, maltodextrin, water.
PHARMACEUTICAL FORM AND CONTENT
Granules for oral suspension - 100 ml plastic bottle with dispenser.
Source Package Leaflet: AIFA (Italian Medicines Agency). Content published in January 2016. The information present may not be up-to-date.
To have access to the most up-to-date version, it is advisable to access the AIFA (Italian Medicines Agency) website. Disclaimer and useful information.
01.0 NAME OF THE MEDICINAL PRODUCT
VECLAM
02.0 QUALITATIVE AND QUANTITATIVE COMPOSITION
- Veclam 250 mg coated tablets:
Each coated tablet contains:
Active principle:
clarithromycin 250 mg.
Sodium content: 3.4 mg per tablet
- Veclam 500 mg coated tablets:
Each coated tablet contains:
Active principle:
clarithromycin 500 mg.
Sodium content: 6.1 mg per tablet
- Veclam 125 mg / 5 ml granules for oral suspension:
100 mL of reconstituted suspension contains:
Active principle:
clarithromycin 2.50 g.
Excipients with known effect: sucrose 550 mg / ml;
castor oil 3.2 mg / ml.
- Veclam 250 mg / 5 ml granules for oral suspension:
100 mL of reconstituted suspension contains:
Active principle:
clarithromycin 5.00 g.
Excipients with known effect: sucrose 455 mg / ml;
castor oil 6.4 mg / ml.
- Veclam 250 mg granules for oral suspension:
Each sachet contains:
Active principle:
clarithromycin 250 mg.
Excipients with known effect: 1591 mg sucrose per sachet;
castor oil 32.1 mg per sachet.
- Veclam 500 mg granules for oral suspension:
Each sachet contains:
Active principle:
clarithromycin 500 mg.
Excipients with known effect: sucrose 3182 mg per sachet;
castor oil 64.2 mg per sachet.
- Veclam 500 mg / 10 ml powder and solvent for solution for infusion:
Each vial of sterile powder for solution for infusion contains:
Active principle
clarithromycin 500 mg.
- Veclam RM 500 mg modified release tablets:
Each modified-release tablet contains:
Active principle:
clarithromycin 500 mg.
Sodium content 15.3 mg per tablet
Excipients with known effect: lactose (115 mg per tablet).
For the full list of excipients see section 6.1.
03.0 PHARMACEUTICAL FORM
- Coated tablets.
- Granules for oral suspension.
- Powder and solvent for solution for infusion.
- Modified release tablets.
04.0 CLINICAL INFORMATION
04.1 Therapeutic indications
Official guidance on the appropriate use of antibacterial agents should be considered.
Veclam is indicated in adults and children over 12 years of age.
Veclam 125 mg / 5 ml granules for oral suspension and Veclam 250 mg / 5 ml granules for oral suspension are indicated in children from 6 months to 12 years of age.
For all pharmaceutical forms:
Treatment of infections caused by pathogens sensitive to clarithromycin. Infections of the rhino-pharyngeal tract (tonsillitis, pharyngitis), of the paranasal sinuses. Lower respiratory tract infections: bronchitis, bacterial pneumonia and atypical pneumonia. Skin infections: impetigo, erysipelas, folliculitis, furunculosis and infected wounds.
Additionally, for Veclam 125 mg / 5 ml granules for oral suspension and for Veclam 250 mg / 5 ml granules for oral suspension:
Acute Otitis Media (AOM).
Additionally for Veclam 250 mg coated tablets and for Veclam 250 mg granules for oral suspension:
Acute and chronic odontostomatological infections sustained by sensitive germs.
In addition, for Veclam 500 mg coated tablets, for Veclam 250 mg granules for oral suspension and for Veclam 500 mg granules for oral suspension:
Localized or diffuse mycobacterial infections caused by Mycobacterium avium or Mycobacterium intracellulare.
Localized infections due to Mycobacterium chelonae, fortuitum or kansasii.
Clarithromycin, in the presence of reduction of gastric acidity, is indicated in the eradication of Helicobacter pylori, producing a consequent decrease in the recurrence of the peptic ulcer.
04.2 Posology and method of administration
In children over 12 years of age: as for adults.
The usual duration of treatment is 5 to 14 days, excluding treatment for community acquired pneumonia and sinusitis which takes 6 to 14 days.
In children under 12 years of age: use Veclam 125 mg / 5 ml granules for oral suspension or Veclam 250 mg / 5 ml granules for oral suspension.
The usual duration of treatment is 5 - 10 days.
The use of Veclam coated tablets, Veclam modified release tablets or Veclam powder and solvent for solution for infusion is not recommended in children below 12 years of age.
Veclam 250 mg coated tablets, Veclam 500 mg coated tablets, Veclam 250 mg granules for oral suspension e Veclam 500 mg granules for oral suspension:
The recommended dose of clarithromycin in adults and children over 12 years of age is 1 tablet or 1 sachet of 250 mg every 12 hours.
In cases of severe infections the dosage can be increased up to 500 mg every 12 hours.
The usual duration of treatment is 5 to 14 days, excluding treatment for community acquired pneumonia and sinusitis which takes 6 to 14 days.
Patients with renal impairment: in patients with renal insufficiency where creatinine clearance is less than 30 ml / min, the dosage should be reduced by half, for example 250 mg once daily or 250 mg twice daily in case of severe infections.
In such patients, administration should not be continued beyond 14 days.
In patients with mycobacterial infections, the starting dose is 500 mg twice daily. If no clinical improvement or bacteriological evidence occurs within 3-4 weeks, the daily dose can be increased to 1000 mg twice daily.
It is recommended, in the treatment of infections spread by Mycobacterium Avium Complex in patients with AIDS, to continue the treatment until clinical or microbiological results are obtained and in any case at the discretion of the treating physician. Clarithromycin should be used in combination with other antimicobacterial drugs .
In odontostomatological infections, the recommended dose is 250 mg every 12 hours for a duration of 5 days.
Dosage schedule in the eradication of Helicobacter pylori:
Triple therapy:
Clarithromycin 500 mg twice daily in combination with omeprazole 20 mg daily and amoxicillin 1000 mg twice daily for 7 to 10 days.
Clarithromycin 500 mg twice daily in combination with lansoprazole 30 mg twice daily and amoxicillin 1000 mg twice daily for 10 days.
Double therapy:
Clarithromycin 500 mg three times daily in combination with omeprazole 40 mg daily for 14 days, followed by omeprazole 20 mg or 40 mg daily for an additional 14 days.
Clarithromycin 500 mg three times daily in combination with lansoprazole 60 mg daily for 14 days. Further suppression of acid secretion may be required for ulcer reduction.
Clarithromycin has also been used in the following therapeutic regimens:
- clarithromycin + tinidazole and omeprazole or lansoprazole
- clarithromycin + metronidazole and omeprazole or lansoprazole
- clarithromycin + tetracycline, subsalicylate bismuth, and ranitidine
- clarithromycin + amoxicillin and lansoprazole
- clarithromycin + ranitidine bismuth citrate
Preparation of the oral suspension in sachets:
To prepare the suspension in sachets, at the time of administration pour the contents of the sachet into a glass of water. Shake until a homogeneous suspension is obtained.
The use of Veclam coated tablets in children under 12 years of age has not been studied.
Veclam RM 500 mg modified release tablets:
The recommended dose of Veclam RM 500 mg modified release tablets in adults and children over 12 years of age is 1 tablet per day to be taken with meals.
In cases of more severe infections, the dosage can be increased to 2 500 mg modified-release tablets per day to be taken as a single dose.
The tablets should be swallowed whole.
The usual duration of treatment is 5 to 14 days, excluding treatment for community acquired pneumonia and sinusitis which takes 6 to 14 days.
Patients with renal impairment: in patients with renal insufficiency with a creatinine clearance value below 30 ml / min, the dosage of clarithromycin should be halved, e.g. 250 mg once a day, or 250 mg twice a day in severe infections. In these patients, treatment should not be continued for more than 14 days. Since the tablet cannot be divided and the 500 mg daily dose cannot be reduced, the modified-release tablet should not be administered to this patient population (see section 4.3).
The use of Veclam modified release tablets in children under 12 years of age has not been studied.
Veclam 125 mg / 5 ml granules for oral suspension and Veclam 250 mg / 5 ml granules for oral suspension:
Clinical studies have been conducted by administering the clarithromycin-based pediatric suspension to children from 6 months to 12 years of age. Consequently, children under 12 years of age should take the pediatric suspension (granules for oral suspension).
The recommended daily dosage in children is 7.5 mg / kg to be administered twice daily for non-mycobacterial infections.
The usual duration of treatment is 5 - 10 days depending on the pathogens involved and the severity of the situation.
The suspension can be taken concomitantly with meals and on an empty stomach and can be swallowed with milk.
Dosing schedule Veclam 125 mg / 5 ml granules for oral suspension
Using the teaspoon
Dosing schedule Veclam 125 mg / 5 ml granules for oral suspension
Use of the dosing syringe for single administration
(Aspirate the suspension up to the corresponding kg mark)
Dosing schedule Veclam 250 mg / 5 ml granules for oral suspension
Using the teaspoon
Dosing schedule Veclam 250 mg / 5 ml granules for oral suspension
Use of the dosing syringe for single administration
(Aspirate the suspension up to the corresponding kg mark)
Preparation of the oral suspension in bottle:
To prepare the Veclam suspension:
- add water to the granules contained in the bottle up to the red line on the bottle.
- Shake well.
- Add more water to bring it back to the line.
The suspension thus prepared has a concentration of 5% for Veclam 250 mg / 5 ml granules for oral suspension and 2.5% for Veclam 125 mg / 5 ml granules for oral suspension and can be stored at room temperature (15 ° C - 30 ° C) for 14 days.
Shake well before each use.
Using the dosing syringe
Unscrew the cap on the bottle. Insert the syringe on the neck of the bottle using the special adapter. Aspirate the desired dose indicated on the dispenser scale and, after having extracted only the syringe, carry out the administration. Then, leaving the adapter inserted, screw the cap back on the neck of the bottle .
The dose should be selected using the scale on the syringe, up to the corresponding body weight of the child.
Veclam 500 mg / 10 ml powder and solvent for solution for infusion:
The recommended dose in adult patients over 18 years of age is 4 - 8 mg / kg / day in two daily administrations. The preparation of the solution to be injected takes place through the dissolution of the powder in the solvent, and the subsequent dilution in 5% glucose or physiological solution, until a final concentration of 1-2 mg / ml is reached.
However, it is advisable not to exceed the maximum dose of 1 g in two daily administrations.
The reconstituted product must be used within 24 hours.
Do not use saline solutions as a solvent. Inject very slowly.
The administration should be continued, depending on the severity of the infection, for up to 6-14 days.
Patients with renal impairment: in patients with renal insufficiency where creatinine clearance is less than 30 ml / min, the dosage should be reduced by half.
In such patients, administration should not be continued beyond 14 days.
There are insufficient data available to recommend a dosage regimen for the use of clarithromycin IV in patients less than 12 years of age (see "Veclam 125 mg / 5 ml granules for oral suspension" and "Veclam 250 mg / 5 ml granules for oral suspension ").
In children between the ages of 12 and 18, the dosage is the same as in adults.
04.3 Contraindications
Hypersensitivity to macrolide class antibiotics or to any of the excipients listed in section 6.1.
- Concomitant administration of clarithromycin with any of the following drugs: astemizole, cisapride, pimozide and terfenadine as they may induce QT interval prolongation and cardiac arrhythmias, including ventricular tachycardia, ventricular fibrillation and torsades de pointes (see section 4.5).
- Concomitant administration of clarithromycin with ticagrelor or ranolazine.
- Concomitant administration of clarithromycin and ergot alkaloids (ergotamine or dihydroergotamine), as it may lead to ergot toxicity (see section 4.5).
- Concomitant administration of clarithromycin and midazolam for oral use (see section 4.5).
Veclam must not be administered to patients with a history of QT interval prolongation or cardiac ventricular arrhythmia, including torsades de pointes (see sections 4.4 and 4.5).
Veclam should not be administered concomitantly with HMG-CoA reductase inhibitors (statins), which are extensively metabolised by CYP3A4 (lovastatin or simvastatin), due to the increased risk of myopathy, including rhabdomyolysis (see section 4.5).
Veclam must not be given to patients with hypokalaemia (risk of QT interval prolongation).
Veclam must not be used in patients suffering from severe hepatic insufficiency associated with kidney damage.
As with other potent inhibitors of the CYP3A4 enzyme, clarithromycin should not be used concomitantly with colchicine (see sections 4.4 and 4.5).
Since the daily dose of 500 mg cannot be reduced, Veclam modified-release tablets are contraindicated in patients with creatinine clearance below 30 ml / min. All other pharmaceutical forms can be used for this patient group.
04.4 Special warnings and appropriate precautions for use
Tablets and Sachets
The use of any antibiotic therapy, such as with clarithromycin, to treat infections with H.pylori it can cause the emergence of resistant bacteria.
All formulations
Clarithromycin should not be prescribed to pregnant women without a "careful benefit / risk assessment, particularly during the first trimester of pregnancy (see section 4.6).
As with other antibiotics, the prolonged use of clarithromycin can cause the onset of superinfections with resistant bacteria and fungi which require the interruption of treatment and the adoption of suitable therapies.
Caution should be exercised in those patients who experience severe renal insufficiency (see section 4.2).
Cases of hepatic dysfunction (see section 4.8) including elevated liver enzymes, hepatocellular damage and / or cholestatic hepatitis, with or without jaundice, have been reported with the use of clarithromycin. This hepatic dysfunction can be severe and is usually reversible. They have been reported. fatal cases of liver failure and have usually been associated with severe underlying disease or concomitant treatments.
The patient should be advised to discontinue treatment and contact their physician if signs and symptoms of liver disease such as anorexia, jaundice, dark urine, itching or abdominal pain occur.
Cases of pseudomembranous colitis, ranging in severity from moderate to life-threatening, have been reported with the use of nearly all antibacterials, including macrolides. Clostridium difficile diarrhea (CDAD) has been reported. with the use of most antibacterials, including clarithromycin, which can range from moderate diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal intestinal flora, which can lead to excessive proliferation of the C. difficult. In all patients who complain of diarrhea after taking antibiotics, the presence of CDAD (Clostridium difficile diarrhea) should be evaluated. These patients should undergo a careful medical history as it has been reported that CDAD can occur during the two months following the intake of antibacterial agents. Therefore, discontinuation of clarithromycin treatment should take place regardless of the therapeutic indication. A microbial test should be performed and appropriate treatment initiated. The administration of antiperistaltic agents should be avoided.
Since clarithromycin is metabolized and excreted mainly in the liver, particular caution should be exercised when administering the drug to patients with impaired hepatic function, in subjects with moderate or severe renal impairment and in the elderly (over 65 years).
Colchicine:
There have been post-marketing reports of colchicine toxicity with the concomitant use of colchicine and clarithromycin, especially in elderly patients, some of which occurred in patients with renal insufficiency. Deaths have been reported in some of these patients (see section 4.5) Concomitant administration of clarithromycin and colchicine is contraindicated (see section 4.3).
Caution is recommended in concomitant administration of clarithromycin and triazolobenzodiazepines, such as triazolam and injectable midazolam (see section 4.5).
Caution is recommended in concomitant administration of clarithromycin and other ototoxic drugs, especially aminoglycosides. It is therefore advisable to periodically monitor vestibular and auditory function during and after treatment.
Due to the risk of QT interval prolongation, clarithromycin should be used with caution in patients with coronary artery disease, severe heart failure, hypomagnesaemia, bradycardia (previous ventricular arrhythmia (see section 4.3).
Pneumonia:
In anticipation of the emerging resistance of the Streptococcus pneumoniae to macrolides, it is important to perform a susceptibility test before prescribing clarithromycin for the treatment of community-acquired pneumonia. In hospital-acquired pneumonia, clarithromycin should be administered in combination with appropriate additional antibiotics.
Skin and soft tissue infections of mild to moderate intensity:
These infections are most often caused by Staphylococcus aureus And Streptococcus pyogenes, both of which may be resistant to macrolides. Then it is necessary to carry out a sensitivity test. In cases where beta-lactam antibiotics cannot be used (e.g. allergies), it is preferable to use other antibiotics, such as clindamycin. Macrolides currently play a fundamental role only in skin and soft tissue infections, such as those caused by Corynebacterium minutissimum, acne vulgaris, erysipelas and in those situations where penicillin-based therapy cannot be established.
In the event of severe acute hypersensitivity reactions such as anaphylaxis, Stevens-Johnson syndrome, toxic epidermal necrolysis and DRESS syndrome, clarithromycin therapy should be discontinued immediately and appropriate treatment adopted immediately.
Veclam should be used with caution when administered concomitantly with medicinal products capable of inducing the CYP3A4 enzyme (see section 4.5).
Attention should be paid to the possibility of cross-resistance between clarithromycin and other macrolides, lincomycin and clindamycin.
HMG-CoA reductase inhibitors (statins): Concomitant use of clarithromycin and lovastatin or simvastatin is contraindicated (see section 4.3). Care should be taken when prescribing clarithromycin with other statins. Rhabdomyolysis has been reported in patients taking clarithromycin and statins. Patients should be monitored for signs and symptoms of myopathy.
In situations where concomitant use of clarithromycin and statins cannot be avoided, it is recommended that the lowest registered dose of statins be prescribed.
The use of a statin that is not dependent on the metabolism of the CYP3A enzyme (eg fluvastatin) may be considered (see section 4.5).
Oral hypoglycemic agents / insulin:
Concomitant use of clarithromycin and oral hypoglycemic agents (such as sulfonylureas) and / or insulin may lead to severe hypoglycaemia. Close glucose monitoring is recommended (see section 4.5).
Oral anticoagulants:
C "is the risk of severe bleeding and a significant increase in the international normalized ratio (INR) and prothrombin time when clarithromycin is co-administered with warfarin (see section 4.5). The" INR and prothrombin time should be frequently monitored in those patients who are treated concomitantly with clarithromycin and oral anticoagulant agents.
Excipients with known effect:
Veclam granules for oral suspension contain sucrose. Patients with rare hereditary problems of fructose intolerance, glucose-galactose malabsorption or sucrase isomaltase insufficiency should not take this medicine.
When prescribing Veclam granules for oral suspension to diabetic patients, the sucrose content should be considered.
Veclam granules for oral suspension also contains castor oil, which can cause stomach upset and diarrhea.
Veclam RM 500 mg modified release tablets contain lactose. Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency, glucose-galactose malabsorption should not take this medicine.
04.5 Interactions with other medicinal products and other forms of interaction
The use of the following medicines is absolutely contraindicated due to the potential serious effects due to their drug interaction.
Cisapride, pimozide, astemizole and terfenadine
Elevated levels of cisapride have been seen in patients taking concomitant cisapride and clarithromycin. Concomitant intake resulted in prolonged QT interval, cardiac arrhythmias including ventricular tachycardia, ventricular fibrillation and torsades de pointes. Similar effects have been observed in patients taking clarithromycin and pimozide concomitantly (see section 4.3).
Macrolides have been reported in the literature to alter the metabolism of terfenadine by increasing its levels which have occasionally been associated with cardiac arrhythmias such as prolonged QT, ventricular tachycardia, ventricular fibrillation and torsades de pointes (see section 4.3). In a study in 14 healthy volunteers, the concomitant administration of clarithromycin and terfenadine resulted in a two to three-fold increase in the serum level of the acid metabolite of terfenadine and a prolongation of the QT interval which did not lead to any detectable clinical effect. Similar effects have been associated with concomitant administration of astemizole and other macrolides.
Alkaloids of ergot
Some post-marketing reports indicate that co-administration of clarithromycin and ergotamine or dihydroergotamine has been associated with acute ergot toxicity (ergotism) characterized by vasospasm and ischaemia of the extremities and other tissues, including the central nervous system. Concomitant administration of clarithromycin and ergot alkaloids is contraindicated (see section 4.3).
HMG-CoA reductase inhibitors (statins)
Concomitant use of clarithromycin and lovastatin or simvastatin is contraindicated (see section 4.3) as these statins are extensively metabolised by CYP3A4 and concomitant treatment with clarithromycin increases their plasma concentration, which increases the risk of myopathy, including rhabdomyolysis.
There have been reports of rhabdomyolysis in patients taking clarithromycin concomitantly with these statins. If treatment with clarithromycin cannot be avoided, therapy with lovastatin or simvastatin should be discontinued during treatment.
Care should be taken when prescribing clarithromycin with statins. In situations where concomitant use of clarithromycin and statins cannot be avoided, it is recommended to prescribe the lowest registered dose of statins. The use of a statin that is not dependent on CYP3A metabolism (eg. fluvastatin). Patients should be monitored for signs and symptoms of myopathy.
Effects of other medicinal products on clarithromycin
Drugs that induce CYP3A (e.g. rifampicin, phenytoin, carbamazepine, phenobarbital, St. John's wort) may induce the metabolism of clarithromycin. This leads to sub-therapeutic levels of clarithromycin with reduced therapeutic efficacy.
In addition, it may be necessary to monitor plasma concentrations of the CYP3A inducer, which may increase due to inhibition of CYP3A by clarithromycin (see also package leaflet of the CYP3A inhibitor administered). Concomitant administration of rifabutin and clarithromycin resulted in an increase in serum levels of rifabutin, a decrease in serum levels of clarithromycin, associated with an increased risk of uveitis.
The following drugs have been known or suspected to affect circulating clarithromycin concentrations; It may be necessary to adjust the dosage of clarithromycin or the possibility of using alternative therapies may be considered.
Efavirenz, nevirapine, rifampin, rifabutin and rifapentine
Drugs that are found to be strong inducers of cytochrome P450 metabolism such as efavirenz, nevirapine, rifampicin, rifabutin and rifapentine may accelerate the metabolism of clarithromycin and consequently lower the plasma levels of clarithromycin while increasing plasma levels. of 14-OH-clarithromycin, a metabolite which is also active from a microbiological point of view. Since the microbiological activities of clarithromycin and 14-OH-clarithromycin are different for different bacteria, the expected therapeutic effect may be nullified during administration concomitant with clarithromycin and enzyme inducers.
Etravirine
Clarithromycin exposure was reduced by etravirine; however, the concentration of the active metabolite, 14-OH-clarithromycin, was increased. Since 14-OH-clarithromycin reduced the activity against the Mycobacterium Avium Complex (MAC), the overall activity against this pathogen may be altered, therefore for the treatment of MAC it is necessary to evaluate alternatives to clarithromycin.
Fluconazole
Concomitant administration of fluconazole 200 mg daily and clarithromycin 500 mg twice daily to 21 healthy volunteers resulted in increases in the mean minimum clarithromycin concentration (Cmin) and area under the curve (AUC). ) of 33% and 18%, respectively. Baseline concentrations of the active metabolite, 14-OH-clarithromycin, were not significantly affected by concomitant administration of fluconazole. No dosage adjustment required for clarithromycin. .
Ritonavir
A pharmacokinetic study has shown that the concomitant administration of 200 mg of ritonavir every 8 hours and 500 mg of clarithromycin every 12 hours leads to marked inhibition of the metabolism of clarithromycin. A 31% increase in clarithromycin Cmax, a 182% increase in Cmin and a 77% increase in AUC were observed with concomitant administration of ritonavir. Complete inhibition of 14-OH-clarithromycin formation was noted. Due to the large therapeutic window of clarithromycin, dose reductions are not necessary in patients with normal renal function. However, in patients with renal insufficiency and concomitant treatment with ritonavir the following dosage adjustment should be considered: if the creatinine clearance (CLCR) is between 30 and 60 ml / minute the dose of clarithromycin should be reduced by 50%; in patients in whom CLCR
Similar dose adjustments should be considered for those patients with impaired renal function administered ritonavir as a pharmacokinetic enhancer of other HIV protease inhibitors, including atazanavir and saquinavir (see Bidirectional Drug Interactions below).
Effects of clarithromycin on other medicinal products
CYP3A based interactions
Concomitant administration of clarithromycin, which is known to inhibit CYP3A, and a drug metabolised primarily by CYP3A, may be associated with increases in drug concentrations which may potentiate or prolong the therapeutic and adverse effects of the drug administered in concomitance.
Clarithromycin should be used with caution in patients receiving other drugs that are thought to be substrates of the CYP3A enzyme, especially if the CYP3A substrate has a narrow margin of safety (e.g. carbamazepine) and / or if the substrate is metabolised. extensively by this enzyme.
Dosage adjustments should be considered and, whenever possible, serum concentrations of drugs metabolised primarily by CYP3A should be carefully monitored in patients receiving concomitant clarithromycin therapy.
Drugs or drug classes known or believed to be metabolised by the same CYP3A isozyme are: alprazolam, oral anticoagulants (e.g. warfarin, see section 4.4), astemizole, carbamazepine, cilostazol, cisapride, cyclosporine, disopyramide, ergot alkaloids, lovastatin, methylprednisolone, midazolam, omeprazole, pimozide, quinidine, rifabutin, sildenafil, simvastatin, sirolimus, tacrolimus, terfenadine, triazolam and vinblastine, but this list is not complete. Other drugs that interact with a similar mechanism through other isozymes within the cytochrome P450 system are phenytoin, theophylline and valproate.
Antiarrhythmics
Post-marketing cases of torsade de pointes have been reported following the concomitant use of clarithromycin and quinidine or disopyramide. During the administration of these drugs concomitantly with clarithromycin it is necessary to monitor the electrocardiographic trace to detect the presence of QT interval prolongation. Monitor serum concentrations of quinidine and disopyramide during use in clarithromycin therapy.
There have been post-marketing reports of hypoglycaemia following the concomitant administration of clarithromycin and disopyramide. Therefore blood glucose levels should be monitored during concomitant administration of clarithromycin and disopyramide.
Oral hypoglycemic agents / Insulin
In the case of concomitant use of clarithromycin with certain hypoglycemic drugs such as nateglinide and repaglinide, inhibition of the CYP3A enzyme by clarithromycin may occur and may cause hypoglycaemia. Close monitoring of glucose levels is recommended.
Omeprazole
Healthy adult subjects received clarithromycin (500 milligrams every 8 hours) in combination with omeprazole (40 milligrams daily). Baseline plasma concentrations of omeprazole were increased (Cmax, AUC0-24, and T½ increased respectively 30%, 89% and 34%) due to the concomitant administration of clarithromycin.
The mean gastric pH value over 24 hours was 5.2 when omeprazole was administered alone, and was 5.7 when omeprazole was administered concomitantly with clarithromycin.
Sildenafil, tadalafil and vardenafil
Each of these phosphodiesterase inhibitors is metabolised, at least partially, by CYP3A and CYP3A may be inhibited by concomitant administration of clarithromycin. Concomitant administration of clarithromycin and sildenafil, tadalafil or vardenafil is very likely to result in increased exposure to the phosphodiesterase inhibitor. Therefore, a reduction in the dosage of sildenafil, tadalafil and vardenafil should be considered when these drugs are co-administered with clarithromycin.
Theophylline, carbamazepine
Results of clinical studies have shown that plasma levels of carbamazepine and theophylline may undergo a modest but statistically significant (p≤0.05) increase when these are co-administered with clarithromycin. A dose reduction may be necessary.
Tolterodina
The major metabolic pathway of tolterodine passes through the 2D6 isoform of cytochrome P450 (CYP2D6). However, in a population subset without CYP2D6, the identified metabolic pathway is CYP3A. In this population subset, CYP3A inhibition. results in significantly higher serum concentrations of tolterodine. In the presence of CYP3A inhibitors, a dose reduction of tolterodine may be necessary as well as a dose reduction of clarithromycin in the patient population in whom CYP2D6 is poorly metabolised.
Triazolobenzodiazepines (eg, alprazolam, midazolam, triazolam)
When midazolam was co-administered with clarithromycin tablets (500 mg twice daily), the AUC of midazolam was increased 2.7-fold following intravenous midazolam administration and 7-fold following intravenous midazolam administration. administration of oral midazolam. Concomitant administration of oral midazolam and clarithromycin should be avoided. In the event that intravenous midazolam is required concomitantly with clarithromycin, the patient should be carefully monitored for a dose adjustment The same precautions should be taken in the presence of other benzodiazepines which are metabolised by CYP3A, including triazolam and alprazolam. In the case of benzodiazepines whose elimination is not dependent on CYP3A (temazepam, nitrazepam, lorazepam), a clinically important interaction with clarithromycin is unlikely.
Drug interactions and central nervous system (CNS) effects (eg somnolence and confusion) have been reported in post-marketing experience with the concomitant use of clarithromycin and triazolam. It is advisable to monitor the patient to keep under control the potential pharmacological effects that this can determine on the Central Nervous System.
Other drug interactions
Aminoglycosides
Care should be taken with the concomitant administration of clarithromycin with other ototoxic drugs, in particular with aminoglycosides (see section 4.4).
Colchicine
Colchicine is a substrate of both CYP3A and the efflux transporter, P-glycoprotein (Pgp). Clarithromycin and other macrolides are known to inhibit CYP3A and Pgp. When clarithromycin and colchicine are administered simultaneously, inhibition of the CYP3A and / or Pgp by clarithromycin may lead to increased exposure to colchicine. Monitor patients for clinical symptoms of colchicine toxicity (see section 4.4).
Digoxin
Digoxin is thought to be a substrate of the efflux transporter, P-glycoprotein (Pgp). Clarithromycin is known to inhibit Pgp. When digoxin and clarithromycin are administered concomitantly, inhibition of Pgp is Some of clarithromycin may lead to increased digoxin exposure. Increases in plasma digoxin concentrations have also been reported during post-marketing surveillance in patients receiving concomitant digoxin and clarithromycin therapy. Some patients have exhibited similar clinical signs. to those presenting with digoxin toxicity, including the onset of life-threatening arrhythmias. Plasma concentrations of digoxin should be closely monitored while patients are receiving concomitant digoxin and clarithromycin therapy.
Zidovudine
Concomitant administration of clarithromycin tablets and zidovudine to adult patients with HIV infections may result in a decrease in steady state zidovudine concentration. Since clarithromycin appears to interfere with the absorption of concomitantly administered orally administered zidovudine, this interaction can be strongly avoided by staggering the doses of clarithromycin and zidovudine to allow an interval of at least 4 hours. This interaction does not appear in pediatric patients with HIV infections. when clarithromycin is taken in granular form at the same time as zidovudine or didanosine This interaction is unlikely when clarithromycin is administered intravenously.
Phenytoin and valproate:
There have been spontaneous or published reports of interactions of CYP3A inhibitors, including clarithromycin, with drugs not considered to be metabolised by CYP3A (e.g. phenytoin and valproate). Serum level determinations are recommended for these drugs when administered concomitantly with clarithromycin. Cases of elevated serum levels have been reported.
Bidirectional Drug Interactions
Atazanavir
Clarithromycin and atazanavir are both substrates and inhibitors of CYP3A and there is evidence of bidirectional drug interaction between these drugs. Concomitant administration of clarithromycin (500 mg twice daily) and atazanavir (400 mg once daily) resulted in a a 2-fold increase in exposure to clarithromycin and a 70% decrease in exposure to 14-OH-clarithromycin with a 28% increase in the AUC of atazanavir. Due to the large therapeutic window of clarithromycin, no dose reduction is required in patients with normal renal function. In the case of patients with moderate renal insufficiency (in which creatinine clearance ranges from 30 to 60 ml / min), the dose of clarithromycin should be reduced by 50%. creatinine is less than 30 ml / min, the clarithromycin dose should be reduced by 75%, using a suitable clarithromycin formulation. The administration of clarithromycin doses greater than 1000 mg per day in conjunction with the administration of protease inhibitors is not recommended.
Calcium channel blockers
Caution is advised in concomitant administration of clarithromycin and calcium channel blockers metabolised by CYP3A4 (e.g. verapamil, amlodipine, diltiazem) due to the risk of hypotension. Plasma concentrations of clarithromycin as well as those of calcium channel blockers may increase due to the interaction. Hypotension, bradyarrhythmia and lactic acidosis have been observed in patients taking clarithromycin and verapamil concomitantly.
Itraconazole
Clarithromycin and itraconazole are both substrates and inhibitors of CYP3A, resulting in a bidirectional drug interaction between these drugs. Clarithromycin may cause an increase in plasma levels of itraconazole while itraconazole may increase plasma levels of clarithromycin.Patients taking clarithromycin and itraconazole concomitantly should be carefully monitored for signs and symptoms of potentiation and prolongation of the pharmacological effects of these drugs.
Saquinavir
Clarithromycin and saquinavir are both substrates and inhibitors of CYP3A, resulting in a "bidirectional drug interaction between these drugs. Concomitant administration of clarithromycin (500 mg twice daily) and saquinavir (soft gelatin capsules, 1200 mg three times per day) in 12 healthy volunteers resulted in saquinavir AUC and Cmax values that were 177% and 187% higher than those seen with saquinavir monotherapy. The AUC and Cmax values of clarithromycin were approximately 40% higher than those seen when clarithromycin monotherapy was administered. No dose adjustment is required when the two drugs are administered concomitantly for a limited period of time at the doses / formulations studied. Observations from drug interaction studies performed using the soft gelatin capsule formulation may not be representative of the effects seen using the saquinavir hard gelatin capsule formulation. Observations from drug interaction studies performed with saquinavir alone may not be representative of the effects seen with saquinavir / ritonavir combination therapy. When saquinavir is administered concomitantly with ritonavir, careful consideration should be given to the potential effects that ritonavir may have on clarithromycin.
04.6 Pregnancy and breastfeeding
Pregnancy
The safety of clarithromycin for use in pregnant women has not been evaluated. Based on the results obtained from studies in mice, rats, rabbits and monkeys, the possibility of harmful effects on embryo-fetal development cannot be excluded. Consequently, use in pregnancy is not recommended without a careful risk / benefit assessment.
Feeding time
The safety of clarithromycin for use during lactation has not been evaluated. Clarithromycin is excreted in breast milk.
04.7 Effects on ability to drive and use machines
There are no data on the effect of clarithromycin on the ability to drive or use machines. The risk of dizziness, vertigo, confusion and disorientation, which may occur following administration, should be considered before the patient drives or uses machines.
04.8 Undesirable effects
to. Summary of the safety profile
The most frequent and common adverse reactions related to clarithromycin therapy for both adult and pediatric patients are abdominal pain, diarrhea, nausea, vomiting and perversion of taste. These adverse events are usually of medium intensity and are consistent with the known safety profile for macrolide antibiotics (see section b of section 4.8).
There is no significant difference in the incidence of these gastrointestinal adverse reactions during clinical trials between patients with or without pre-existing mycobacterial infections.
b. Summary table of adverse reactions
The following table summarizes the adverse reactions reported during clinical studies and post-marketing experience with clarithromycin immediate release tablets, granules for oral suspension, powder and solvent for solution for infusion and modified release tablets.
Adverse reactions considered possibly related to clarithromycin are reported by organ type and frequency, according to the following convention: very common (≥1 / 10), common (≥1 / 100,
* Since these reactions have been reported voluntarily from a population of an indefinite size, it is not always possible to make a true estimate of the frequency or establish a cause-and-effect relationship with drug exposure. patient exposure exceeds one billion days of patient treatment with clarithromycin
** In some of the reported cases of rhabdomyolysis, clarithromycin was administered concomitantly with statins, fibrates, colchicine or allopurinol.
1 Adverse reaction reported for powder and solvent formulation for solution for infusion only
2 Adverse reaction reported for granules for oral suspension only
3 Adverse reaction reported for immediate release tablet formulation only
4, 6, 8,9 See paragraph a)
5, 7, 10 See paragraph c)
c. Description of selected adverse reactions
Injection site phlebitis, injection site pain, needle stick pain and injection site inflammation are specific to the intravenous formulation.
In some of the reported cases of rhabdomyolysis, clarithromycin was administered concomitantly with statins, fibrates, colchicine or allopurinol (see sections 4.3 and 4.4).
There have been post-marketing reports of drug interactions and Central Nervous System (CNS) effects (eg somnolence and confusion) with the concomitant use of clarithromycin and triazolam. It is suggested that the patient be monitored for increased pharmacological effects at CNS level (see section 4.5).
Rare cases of clarithromycin modified release tablets in faeces have been reported, most of which occurred in patients with anatomical changes (including ileostomy or colostomy) or gastrointestinal function disorders with shortened gastrointestinal transit time. In several cases, tablet residues have occurred in the context of diarrhea. For those patients who have experienced the presence of tablet residues in the stool and no improvement in their condition, a change to a different formulation of clarithromycin (e.g. oral suspension) or another antibiotic is recommended.
Special population: Adverse reactions in immunocompromised patients (see section e).
d. Pediatric populations
Clinical studies have been conducted by administering the clarithromycin-based pediatric suspension to children from 6 months to 12 years of age. Consequently, children under 12 years of age should take the pediatric suspension. There are insufficient data available to recommend a dosage regimen for the use of clarithromycin IV in patients less than 12 years of age.
The frequency, type and severity of adverse reactions are expected to be comparable to those occurring in adults.
And. Other special populations
Immunocompromised patients
In AIDS or immunocompromised patients treated for mycobacterial infections with high doses of clarithromycin for long periods it has often been difficult to distinguish adverse reactions possibly associated with clarithromycin administration from human immunodeficiency virus (HIV) or intercurrent disease associated manifestations. .
In adult patients, the most frequently reported adverse reactions by patients treated with total daily doses of 1000 mg and 2000 mg clarithromycin were: nausea, vomiting, taste perversion, abdominal pain, diarrhea, rash, flatulence, headache , constipation, impaired hearing, increased serum Glutamic-Oxaloacetic Transaminase (SGOT) and Serum Glutamic-Pyruvic Transaminase (SGPT). Additional less frequent reactions include dyspnoea, insomnia and dry mouth. The incidence was comparable for those patients treated with 1000 mg and 2000 mg, but were generally 3 to 4 times more frequent in those patients receiving a total daily dose of clarithromycin of 4000 mg.
In these immunocompromised patients, evaluations of laboratory values were made by analyzing those values outside the abnormal levels considered serious for the specific test (eg, upper and lower limits). Based on these criteria, approximately 2% or 3% of patients who took 1000 mg or 2000 mg of clarithromycin daily had extremely high abnormal SGOT and SGPT values, and extremely low white blood cell and platelet counts. A lower percentage of patients included in these two dose groups also showed elevated BUN values. A slightly higher incidence of abnormal values was noted in patients treated with 4000 mg of clarithromycin daily for all parameters excluding the leukocyte formula.
Reporting of suspected adverse reactions.
Reporting of suspected adverse reactions occurring after authorization of the medicinal product is important as it allows continuous monitoring of the benefit / risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system. "address https://www.aifa.gov.it/content/segnalazioni-reazioni-avverse.
04.9 Overdose
In case of high doses of clarithromycin gastrointestinal disturbances can occur. A patient suffering from bipolar disorder ingested eight grams of clarithromycin showing altered mental status, paranoid behavior, hypokalaemia, hypoxemia. Adverse reactions occurring in overdose should be treated with immediate elimination of the unabsorbed drug and appropriate supportive care. As with other macrolides, clarithromycin serum levels are not eliminated by hemodialysis or peritoneal dialysis.
In the event of an overdose, treatment with clarithromycin IV (powder and solvent for solution for infusion) should be discontinued and all appropriate supportive measures instituted.
05.0 PHARMACOLOGICAL PROPERTIES
05.1 Pharmacodynamic properties
Pharmacotherapeutic group: general antibacterials for systemic use - macrolides.
ATC code: J01FA09.
Clarithromycin is a new macrolide developed by Abbott, resulting from the substitution at position 6 in the lactone ring of erythromycin of a hydroxyl group with the CH3O group.
The new macrolide has been shown to possess in vitro an antibacterial spectrum active against the best known and clinically important both Gram positive and Gram negative bacteria, including aerobes and anaerobes.
The in vitro antibacterial spectrum of clarithromycin was shown to be as follows: Streptococcus agalactiae, Streptococcus pyogenes, Streptococcus viridans, Streptococcus pneumoniae, Haemophilus influenzae, Haemophilus parainfluenzae, Neisseria gonorrheaopleae, Listeria monocyasmae, Chacterionella pneumoniajunijuni, Chacterionella pneumoniaj , Branhamella catharralis, Bordetella pertussis, Staphilococco aureus, Propionibacterium acnes, Mycobacterium avium, Mycobacterium leprae, Mycobacterium intracellulare, Mycobacterium chelonae, Mycobacterium fortuitum and Mycobacterium kansasii.
Its action takes place by binding with the 50S ribosomal subunit, inhibiting the protein synthesis of the bacterial cell.
BREAKPOINTS
The European Committee for Antimicrobial Sensitivity Tests (EUCAST) has established the following breakpoints for clarithromycin, separating susceptible organisms.
Clarithromycin is used for the "eradication of"H. pylori: minimum inhibitory concentration (MIC) ≤ 0.25 mcg / ml which has been established as a sensitivity breakpoint by the Clinical and Laboratory Standards Institute (C.I.S.I).
05.2 Pharmacokinetic properties
Studies in dogs have shown that after intravenous or oral administration of 10 mg / kg there were plasma drug concentrations of 3, 2 or 1 mg / ml at 1, 4 and 12 hours, respectively.
Within 5 days of oral or intravenous administration of (14C) -labelled clarithromycin, approximately 35-36% of the 14C dose was recovered as is in the urine and approximately 52% in the faeces.
Clarithromycin is metabolised in the liver and the most important metabolite is 14-hydroxy-N-demethyl clarithromycin which reaches peak plasma concentrations of 0.5 mcg / ml and 1.2 mcg / ml after 2-4 hours after administration of 250 and 1200 mg. Only after oral intake of 1200 mg were also low levels of descladinosil-clarithromycin identified in the plasma; the metabolic process tends to saturation at high doses.
Pharmacokinetic studies in humans have shown peak plasma concentrations of 2.08 μg / ml after oral administration of 250 mg of clarithromycin.
Following intravenous administration of 500 mg clarithromycin mean plasma peaks of 5.52 ± 0.98 mcg / ml are achieved.
The half-life of the compound is equal to 6.3 hours.
The same metabolites that are formed following oral administration are identified, but in lower concentrations, presumably in relation to the absence of a first pass hepatic metabolism.
Modified release formulation:
The pharmacokinetics of modified release clarithromycin administered orally were studied in adult patients and compared with clarithromycin 250 mg and 500 mg immediate release tablets. When equal total daily doses were administered, the extent of absorption was equivalent. Absolute bioavailability is approximately 50%.
Following multiple dosing, slight accumulation was found and metabolism did not change in any species.
Based on the equivalent absorption results, the following data of the modified release formulation are applicable in vitro and in vivo.
In vitro
In vitro studies have shown that the protein binding of clarithromycin in human plasma averages about 70%. at concentrations of 0.45 - 4.5 mcg / ml. A decrease in binding to 41% at a concentration of 45 mcg / ml suggests that the binding sites could become saturated, however this only occurred at high drug concentrations far from therapeutic levels.
In vivo
In all tissues, clarithromycin concentrations, excluding the central nervous system, were much higher than circulating drug concentrations.
The highest concentrations were found in the liver and lung tissue, where the tissue / plasma ratio was 10 to 20.
The pharmacokinetic behavior of clarithromycin is not linear. In patients who ate and received modified-release clarithromycin 500mg / day, peak steady-state plasma concentrations of clarithromycin and 14-OH clarithromycin were 1.3 mcg / ml and 0.48, respectively. mcg / ml.When the dosage was increased up to 1000 mg / day, the steady-state concentration values were 2.4 mcg / mL and 0.67 mcg / mL, respectively.
Clarithromycin is metabolised in the liver by cytochrome P450. Three metabolites have been described: N-demethyl-clarithromycin; decladinosil-clarithromycin and 14-hydroxy-clarithromycin.
The elimination half-lives of clarithromycin and its active metabolite were 5.3 and 7.7 hours, respectively.
At higher concentrations, the apparent half-life of both clarithromycin and its metabolite tends to be longer.
Clarithromycin is excreted via the urine (approx. 40%) and faecally (approx. 30%).
05.3 Preclinical safety data
The LD50 in mice and rats was greater than 5 g / kg orally and greater than 300 mg / kg orally in dogs and monkeys. Short-term toxicity (1 month) showed no toxic effects, neither on rats (150 mg / kg / day), nor on dogs (10 mg / kg / day). Furthermore, chronic toxicity (3 months) was found to be 15 mg / kg / day in rats and 10 mg / kg / day in dogs.
Mutagenicity tests have shown that the drug has no mutagenic effects or microsomal activation. Clarithromycin had no effect on the motor activity of the mouse after oral administration of 100 mg / kg.
06.0 PHARMACEUTICAL INFORMATION
06.1 Excipients
Veclam 250 mg coated tablets:
Croscarmellose sodium, pregelatinized starch, microcrystalline cellulose, E-104, silica gel, povidone, stearic acid, magnesium stearate, talc, hypromellose, propylene glycol, sorbitan monoleate, vanillin, E-171, hydroxypropyl cellulose, sorbic acid.
- Veclam 500 mg coated tablets:
Croscarmellose sodium, microcrystalline cellulose, silica gel, povidone, stearic acid, magnesium stearate, talc; coating solution: hypromellose, hydroxypropylcellulose, propylene glycol, sorbitan monoleate, E-171, sorbic acid, vanillin, E-104.
- Veclam 125 mg / 5 ml granules for oral suspension and Veclam 250 mg / 5 ml granules for oral suspension:
Carbopol 974, povidone, hypromellose phthalate, castor oil, silica gel, sucrose, xanthan gum, mixed fruit flavor, potassium sorbate, citric acid, titanium dioxide, maltodextrin, water.
- Veclam 500 mg / 10 ml powder and solvent for solution for infusion:
Lactobionic acid, sodium hydroxide as a pH adjuster.
Each solvent vial contains:
Water p.p.i.
- Veclam 250 mg granules for oral suspension and Veclam 500 mg granules for oral suspension:
Carbopol 974P, povidone K90, hydroxypropyl methylcellulose phthalate, castor oil, silicon dioxide, maltodextrin, sucrose, titanium dioxide, modified starch, orange flavor, glycyrizinated ammonium, acesulfame K.
- Veclam RM 500 mg modified release tablets:
Anhydrous citric acid, sodium alginate, sodium and calcium alginate, lactose, povidone K30, talc, stearic acid, magnesium stearate, hypromellose 6cps, macrogol 400, macrogol 8000, titanium dioxide (E-171), sorbic acid, quinoline yellow (E -104).
06.2 Incompatibility
There are currently no specific incompatibilities with known drugs.
06.3 Period of validity
Veclam 250 mg coated tablets 3 years.
Veclam 500 mg coated tablets 3 years.
Veclam 125 mg / 5 ml granules for oral suspension 2 years.
Veclam 250 mg / 5 ml granules for oral suspension 2 years.
Veclam 250 mg granules for oral suspension 3 years.
Veclam 500 mg granules for oral suspension 3 years.
Veclam 500 mg / 5 ml powder and solvent for solution for infusion 3 years.
Veclam RM 500 mg modified release tablets 3 years.
06.4 Special precautions for storage
For the package 500 mg granules for oral suspension: Store at a temperature not exceeding 25 ° C.
For pack sizes 250 mg coated tablets, 500 mg coated tablets, RM 500 modified release tablets, 250 mg granules for oral suspension, 125 mg / 5 ml granules for oral suspension and 250 mg / 5 ml granules for oral suspension: This medicinal product does not requires no special storage conditions.
For the pack 500 mg / 10 ml powder and solvent for solution for infusion: No special precautions for storage. The reconstituted product must be used within 24 hours.
06.5 Nature of the immediate packaging and contents of the package
- Veclam 250 mg coated tablets:
Cardboard box containing 12-cell opaque blisters
- Veclam 500 mg coated tablets:
Cardboard box containing 14-cell opaque blister
- Veclam RM 500 mg modified release tablets:
Cardboard box containing blister packs of 7 cells
- Veclam 125 mg / 5 ml granules for oral suspension:
100 ml plastic bottle with dispenser
- Veclam 250 mg / 5 ml granules for oral suspension:
100 ml plastic bottle with dispenser
- Veclam 500 mg / 10 ml powder and solvent for solution for infusion:
Cardboard box containing 1 ampoule of 500 mg of clarithromycin + 1 ampoule of solvent
- Veclam 250 mg granules for oral suspension:
Cardboard box containing 14 sachets of 250 mg
- Veclam 500 mg granules for oral suspension:
Cardboard box containing 14 sachets of 500 mg
06.6 Instructions for use and handling
See section 4.2
07.0 MARKETING AUTHORIZATION HOLDER
MALESCI Pharmacobiological Institute S.p.A. Via Lungo l "Ema, 7 - Bagno a Ripoli (FI)
Under license from ABBOTT S.r.l. - Campoverde di Aprilia (LT)
08.0 MARKETING AUTHORIZATION NUMBER
Veclam 250 mg coated tablets:
Blister 12 tablets - AIC 027529054
Veclam 500 mg coated tablets:
Blister 14 tablets - AIC 027529116
Veclam 125 mg / 5 ml granules for oral suspension:
100 ml bottle - AIC 027529041
Veclam 250 mg / 5 ml granules for oral suspension:
100 ml bottle - AIC 027529104
Veclam 250 mg granules for oral suspension:
14 Sachets - AIC 027529080
Veclam 500 mg granules for oral suspension:
14 Sachets - AIC 027529092
Veclam 500 mg / 10 ml powder and solvent for solution for infusion:
Bottle + solvent vial - AIC 027529039
Veclam RM 500 mg modified release tablets:
Blister of 7 modified release tablets - AIC n. 027529130
09.0 DATE OF FIRST AUTHORIZATION OR RENEWAL OF THE AUTHORIZATION
Veclam 250 mg coated tablets 02/19/1997
Veclam 500 mg coated tablets 06/03/1999
Veclam 125 mg / 5 ml granules for oral suspension 02/19/1997
Veclam 500 mg / 10ml powder and solvent for solution for infusion 01/04/1992
Veclam 250 mg / 5 ml granules for oral suspension 17/08/1999
Veclam 250 mg granules for oral suspension 17/03/1999
Veclam 500 mg granules for oral suspension 03/17/1999
Veclam RM 500 mg modified release tablets 30/05/2001
Renewal of the authorization: 31/05/2010
10.0 DATE OF REVISION OF THE TEXT
AIFA Determination of April 2015