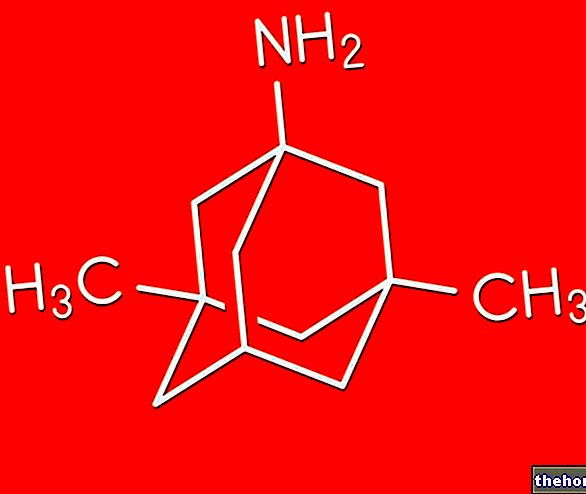
Active ingredients: Prulifloxacin
KERAFLOX 600 mg film-coated tablets
Why is Keraflox used? What is it for?
Keraflox belongs to a group of antibiotics called fluoroquinolones. Keraflox is indicated for the treatment of infections caused by bacteria sensitive to prulifloxacin in the following conditions:
- Lower urinary tract infections (simple cystitis).
- Lower urinary tract infections associated with other medical urinary complications (complicated cystitis).
- Sudden aggravation of chronic bronchitis (flare-up of chronic bronchitis).
- Acute bacterial rhinosinusitis.
The doctor will diagnose and treat infectious rhinosinusitis according to national and local guidelines on the treatment of infections. Keraflox can be used to treat infectious rhinosinusitis whose symptoms last less than 4 weeks, and in cases where normal antibiotics cannot be used or have not worked.
Contraindications When Keraflox should not be used
Do not take Keraflox:
- If you are allergic to prulifloxacin, other fluoroquinolones or any of the other ingredients of this medicine.
- If you are under the age of 18.
- If you have already suffered from tendon problems after using other quinolones, such as inflammation of the tendons (tendonitis).
- If you are pregnant or breastfeeding.
Precautions for use What you need to know before taking Keraflox
Talk to your doctor or pharmacist before taking Keraflox:
- If you have epilepsy or a disease that makes it more likely that you will have convulsions (fits)
- Since heart rhythm changes (seen on ECG, a recording of the heart's electrical activity) have been seen with other antibiotics belonging to the fluoroquinolone class, please tell your doctor if you have a history of heart rhythm disturbances. Keraflox has a very low potential for QT interval prolongation induction
- If you are taking medicines to control heart rhythm or medicines that may have cardiac effects such as antidepressants or other antibiotics (see "Taking Keraflox with other medicines")
- If you suffer from glucose-6-phosphate dehydrogenase (G6PD) activity deficiency, as this drug may not be suitable
- If you have liver or kidney problems
- If you suffer from lactose intolerance, as this medicine contains lactose
- If you have experienced severe bouts of diarrhea following antibiotic use. Tell your doctor immediately and stop taking Keraflox if you experience a severe bout of liquid diarrhea while taking Keraflox. black-tar or containing blood.
This medicine can sometimes cause muscle or tendon problems (see 'Possible side effects').
Tell your doctor immediately and stop taking Keraflox if you experience muscle pain, muscle weakness, dark urine or symptoms of tendon inflammation such as joint swelling or pain while taking Keraflox. affected should be kept at rest until the doctor has examined them.
Since this drug can cause the formation of tiny crystals in the urine, in order to prevent urine concentration it is necessary to maintain a high water intake during treatment with Keraflox.
Excessive exposure to the sun, ultraviolet lamps or sun beds should be avoided during treatment with this medicine as the skin may be more sensitive than normal. Stop taking this medicine and tell your doctor immediately if you experience severe reactions to the sun such as sunburn or skinning.
If your vision decreases or if your eyes are otherwise impaired, consult an ophthalmologist immediately.
Interactions Which drugs or foods can modify the effect of Keraflox
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Some drugs affect the effects of Keraflox. Keraflox should be taken 2 hours before or at least 4 hours after taking these medicines.
- Medicines for indigestion, heartburn or ulcers, such as cimetidine or antacids containing aluminum or magnesium
- Medicines containing iron or calcium
Keraflox in turn can affect the effects of other medicines and increase the risk of side effects.
Tell your doctor if you are taking:
- Medicines for diabetes
- Medicines to control heart rate such as amiodarone, quinidine or procainamide
- Other antibiotics such as erythromycin, clarithromycin or azithromycin
- Medicines for depression such as amitriptyline, clomipramine or imipramine
- Probenecid to reduce uric acid in the blood
- Fenbufen to relieve arthritis pain
- Theophylline for asthma or breathing difficulties
- Medicines to prevent blood clotting such as warfarin
- Nicardipine used to treat angina (chest pain) or high blood pressure
- Steroids such as prednisolone used to treat allergic states or inflammation
Keraflox with food and drink
Food and milk can influence the effects of Keraflox. Keraflox should be taken between meals on an empty stomach and should not be taken with milk or milk derivatives.
Warnings It is important to know that:
Pregnancy, breastfeeding and fertility
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Driving and using machines
Keraflox can cause dizziness and confusion. If you experience any of these symptoms, do not drive or use any dangerous tools or machinery.
Keraflox contains lactose
Keraflox contains lactose, a type of sugar. If you have been told by your doctor that you have "intolerance to some sugars, contact your doctor before taking this medicinal product.
Dose, Method and Time of Administration How to use Keraflox: Posology
Always take this medicine exactly as your doctor has told you. If in doubt, consult your doctor or pharmacist.
Keraflox tablets should be swallowed whole with water and between meals on an empty stomach. They should not be taken with milk or milk derivatives.
Keraflox is for adults only. The recommended dose is:
- For simple cystitis: one 600 mg tablet once.
- For complicated cystitis: one 600 mg tablet once daily for up to 10 days of treatment.
- For the exacerbation of chronic bronchitis: one 600 mg tablet once a day for a maximum of 10 days of treatment.
- For acute bacterial rhinosinusitis: one 600 mg tablet once daily for up to 10 days of treatment.
It is necessary to drink plenty of water while taking Keraflox.
The duration of treatment depends on the severity of the infection and the patient's response to treatment. You should always complete the full course of tablets prescribed for you even if you begin to feel better and your symptoms disappear.
If you forget to take Keraflox
If you forget to take a dose, take it as soon as you remember unless it is already time for your next dose. Do not take a double dose to make up for a forgotten dose.
If you stop taking Keraflox
If you stop taking this medicine too soon, the infection may come back. If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
Overdose What to do if you have taken too much Keraflox
In the event of an overdose, contact your doctor immediately or go to the nearest hospital emergency department. Your hospital doctor may need to perform a stomach emptying procedure. Always carry the package with the package leaflet with you, whether or not there is still Keraflox left in the package.
Side Effects What are the side effects of Keraflox
Like all medicines, Keraflox can cause side effects, although not everybody gets them.
Tell your doctor immediately and stop taking Keraflox if you experience any of the following symptoms after taking this medicine.
Although very rare, these symptoms can be severe.
- Sudden wheezing, difficulty in breathing, swelling of the eyelids, face or lips, rash or itching (especially all over the body).
- Severe rash involving blisters on the skin and sometimes in the mouth and tongue. These could be symptoms of a condition known as Stevens Johnson Syndrome.
- Severe solar reactions such as sunburn or peeling.
- Symptoms of inflammation of the tendons such as swelling or pain of the affected limb. Most frequently it affects the Achilles tendon and can lead to its rupture. The part affected by the inflammation should be kept at rest until the doctor has examined it. .
- Muscle pain, muscle weakness, or dark urine.
- Severe bouts of liquid diarrhea that is tar-black or bloody.
- Low blood sugar levels which can cause tremor and irritability.
- Numbness, loss of pain sensation.
- Redness and peeling of the skin (dermatitis).
- Formation of tiny crystals in the urine in the absence of symptoms.
Other possible side effects are:
Common side effects (in less than one in 10 patients):
- Abdominal pain
Uncommon side effects (in less than one in 100 patients):
- Feeling unwell
- Diarrhea, vomiting, stomach inflammation
- Headache, dizziness
- Itching or rash
- Loss of appetite
Rare side effects (in less than one in 1000 patients):
- Fever, hot flashes
- Changes in taste
- Sleep disturbances, confusion or sleepiness
- Hearing reduction
- Redness and irritation of the eyes
- Stomach pain, wind, bloating, indigestion or heartburn, abnormal stools
- Irritation of the lips, tongue or mouth, or fungal infection (oral moniliasis)
- Muscle spasms, muscle damage
- Dry and itchy skin (eczema), hypersensitivity to light or red spots on the skin (hives)
- Increased liver enzymes visible in blood tests
- Feeling restless
- Mouth ulcer
- Joint pain spread over the whole body
- Increased levels of albumin (protein) in the blood
- Increased levels of calcium in the blood
- Increase in the number of white blood cells
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system at www.agenziafarmaco.it/it/responsabili. By reporting side effects you can help provide more information on the safety of this medicine.
Expiry and Retention
Keep this medicine out of the sight and reach of children.
Do not store above 30 ° C.
Store in the original container.
Do not use Keraflox after the expiry date which is stated on the pack. The expiry date refers to the last day of the month.
Do not throw any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. This will help protect the environment.
Other information
What Keraflox contains
The active substance is prulifloxacin.Each film-coated tablet contains 600 mg of prulifloxacin.
The other ingredients are: lactose monohydrate; microcrystalline cellulose; croscarmellose sodium; povidone; anhydrous colloidal silica; magnesium stearate; hypromellose; propylene glycol; titanium dioxide (E171); talc; ferric oxide (E 172).
Description of what Keraflox looks like and contents of the pack
Keraflox tablets are yellow, oblong, film-coated and are available in carton packs containing one blister of 1, 2, 5 tablets or two blisters of 5 tablets.
Not all pack sizes may be marketed.
Source Package Leaflet: AIFA (Italian Medicines Agency). Content published in January 2016. The information present may not be up-to-date.
To have access to the most up-to-date version, it is advisable to access the AIFA (Italian Medicines Agency) website. Disclaimer and useful information.
01.0 NAME OF THE MEDICINAL PRODUCT
KERAFLOX 600 MG TABLETS COATED WITH FILM
02.0 QUALITATIVE AND QUANTITATIVE COMPOSITION
Each film-coated tablet contains 600 mg of Prulifloxacin
Excipients with known effect: each film-coated tablet contains 76 mg of lactose
For the full list of excipients see section 6.1.
03.0 PHARMACEUTICAL FORM
Film-coated tablets.
Oblong, yellow, film-coated tablets.
04.0 CLINICAL INFORMATION
04.1 Therapeutic indications
Keraflox is indicated for the treatment of infections caused by susceptible strains, in the following pathologies:
• acute uncomplicated infections of the lower urinary tract (simple cystitis);
• complicated lower urinary tract infections;
• exacerbation of chronic bronchitis;
• acute bacterial rhinosinusitis.
Acute bacterial sinusitis must be adequately diagnosed in accordance with national or local guidelines on the treatment of respiratory infections.For the treatment of bacterial rhinosinusitis, Keraflox should only be used in patients in whom the duration of symptoms is less than 4 weeks and when the use of other antibacterials commonly recommended for the initial treatment of such infection is considered inappropriate, or in the case of which these were found to be ineffective.
In the treatment of patients with infectious diseases, local characteristics relating to antibiotic sensitivity must be taken into account.
04.2 Posology and method of administration
Dosage
Limited to adults, the indicative dosage is as follows:
• patients with uncomplicated acute infections of the lower urinary tract (simple cystitis): only one 600 mg tablet is sufficient;
• patients with complicated lower urinary tract infections: one 600 mg tablet once daily for up to 10 days of treatment.
• patients with exacerbation of bronchitis: one 600 mg tablet once a day for a maximum of 10 days of treatment.
• patients with acute bacterial rhinosinusitis: one 600 mg tablet once a day for a maximum of 10 days of treatment.
In the case of complicated lower urinary tract infections and acute exacerbation of chronic bronchitis, the duration of treatment depends on the severity of the disease and the clinical course of the patient and must in any case continue for at least 48-72 hours from the remission / disappearance of symptoms.
Due to the lack of specific studies it is not possible to determine the posology in patients with renal insufficiency (patients with creatinine clearance hepatic insufficiency. Therefore, in these patients the monitoring of the plasma levels of the drug is the most reliable method for dosage adjustment.
Method of administration
Keraflox tablets should be swallowed whole with water and should be administered according to food intake (see section 4.5).
04.3 Contraindications
- Hypersensitivity to prulifloxacin, to other quinolone antibacterials or to any of the excipients listed in section 6.1.
- Children before pubertal age or boys under the age of 18 with incomplete skeletal development.
- Patients with a history of tendon diseases related to the administration of quinolones.
- Pregnancy and lactation (see section 4.6).
04.4 Special warnings and appropriate precautions for use
As with other quinolones, Keraflox should be used with caution in patients with CNS disorders that may predispose to seizures or lower the seizure threshold.
Some of the other substances belonging to the fluoroquinolone class have been associated with cases of QT interval prolongation. Prulifloxacin has a very low potential for induction of QT interval prolongation.
As with the administration of other drugs of the same therapeutic class, tendonitis occurs rarely. Most frequently it affects the Achilles tendon and can lead to its rupture. The risk of tendonitis and tendon ruptures is increased in elderly patients and in patients receiving corticosteroids.
Patients should be advised, in the event of signs of tendon inflammation, myalgia, pain or joint inflammation, to discontinue treatment and to keep the affected limb or limbs at rest until the diagnosis of tendonitis is been excluded.
Exposure to the sun or ultraviolet rays may cause phototoxicity in patients being treated with prulifloxacin, as well as with other quinolones. Excessive exposure to the sun or ultraviolet rays should be avoided during treatment with Keraflox; in the event of phototoxicity, the treatment should be discontinued.
Patients with latent or known defects in glucose-6-phosphate dehydrogenase activity are prone to haemolytic reactions when treated with quinolone antibacterials and for this reason Keraflox should be used with caution.
As reported for other quinolones, rhabdomyolysis phenomena may rarely occur, characterized by myalgia, asthenia, increased plasma CPK and myoglobin values, and rapid deterioration of renal function. In these cases, the patient should be carefully monitored and appropriate corrective measures taken, including possibly stopping treatment.
The use of quinolones is sometimes related to the appearance of crystalluria; patients treated with this class of products must maintain an adequate water balance in order to avoid urine concentration.
The tolerability and efficacy of Keraflox in patients with hepatic insufficiency has not been evaluated.
Local and / or national guidelines on the appropriate use of antibacterials should be considered when prescribing antibiotic therapy.
The medicine contains lactose; therefore patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicine.
Vision disorders
If vision becomes impaired or if any effects on the eyes occur, an ophthalmologist should be consulted immediately.
Disease associated with Clostridium difficile
If diarrhea occurs during or after prulifloxacin therapy (even several weeks after treatment), particularly if it is severe, persistent and / or bleeding, it could be a result of disease associated with Clostridium difficile (Clostridium difficile-associated disease, CDAD). The severity of CDAD can range from mild to life-threatening; the most severe form is pseudomembranous colitis (see section 4.8). Therefore, it is important to consider this diagnosis in patients who develop severe diarrhea during or after treatment with prulifloxacin. If CDAD is suspected or confirmed, ongoing treatment with antibacterial agents, including prulifloxacin, should be discontinued immediately and appropriate therapy initiated promptly. In this clinical condition, anti-peristaltic medicinal products are contraindicated. In addition, to reduce the risk of transmission, adequate infection control measures must be taken.
04.5 Interactions with other medicinal products and other forms of interaction
Concomitant treatment with cimetidine, antacids containing Al and Mg or preparations containing iron and calcium reduces the absorption of Keraflox; consequently Keraflox should be administered 2 hours before or at least 4 hours after taking these preparations.
The concomitant intake of prulifloxacin and milk causes a decrease in the area under the concentration / time curve (AUC) and reduces the urinary elimination of prulifloxacin, while the ingestion of food slows down and reduces peak levels.
The urinary excretion of prulifloxacin decreases when given together with probenecid. Concomitant administration of fenbufen with some quinolones may result in an increased risk of seizures, therefore the administration of Keraflox and fenbufen should be carefully considered.
Quinolones can cause hypoglycemia in diabetic patients taking hypoglycemic drugs. Concomitant administration of Keraflox and theophylline may cause a slight decrease in theophylline clearance which is not expected to have any clinical relevance. However, as with other quinolones, monitoring of plasma theophylline levels is advisable in patients with metabolic disorders or who have risk factors.
Quinolones can enhance the effects of oral anticoagulants such as warfarin and its derivatives; if these products are co-administered with Keraflox, close monitoring with prothrombin test or other reliable coagulation tests is recommended.
Preclinical data have shown that nicardipine can potentiate the phototoxicity of prulifloxacin. No clinically significant interactions were observed during the clinical development of Keraflox following concomitant administration with other medicinal products commonly used in the treatment of patients with the conditions listed in section 4.1.
04.6 Pregnancy and breastfeeding
Pregnancy
No clinical data are available on the use of prulifloxacin during established pregnancy. Animal studies did not indicate teratogenicity. Other toxic effects on reproduction were only detected in maternal toxicity (see section 5.3).
Feeding time
In rats, prulifloxacin was shown to cross the placental barrier and pass in large quantities into breast milk. As with other quinolones, prulifloxacin has been shown to cause arthropathies in young animals, and therefore its use during pregnancy and lactation is contraindicated.
04.7 Effects on ability to drive and use machines
Quinolones can cause dizziness and confusion, therefore, the patient should know how he responds to treatment before driving or operating machinery or engaging in activities that require alertness and coordination.
04.8 Undesirable effects
The undesirable effects listed below are attributable to clinical studies carried out with Keraflox. Most adverse events were mild or moderate in intensity.
The following MedDRA frequency values were used: very common (≥ 1/10), common (≥ 1/100,
The following adverse reactions have also been reported (frequency not known): anaphylactic / anaphylactoid reaction including angioedema, dyspnoea, Steven Johnson syndrome, hypoglycemia, hypoesthesia, paraesthesia, tremor, drug dermatitis, rhabdomyolysis, phototoxicity, tachycardia, pseudomembranous colitis.
Treatment with Keraflox may be associated with asymptomatic crystalluria without changes in creatinine levels, with changes in liver function parameters and eosinophilia. In the cases observed, these changes were asymptomatic and transient.
During treatment with Keraflox, the occurrence of adverse reactions and laboratory abnormalities not mentioned above, but reported for the other quinolones, cannot be excluded.
Pharmacovigilance data for prulifloxacin and post-marketing show sporadic reports of tendinopathy (see 4.4 Special warnings and precautions for use).
Reporting of suspected adverse reactions
Reporting of suspected adverse reactions occurring after authorization of the medicinal product is important as it allows continuous monitoring of the benefit / risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system. "address www.agenziafarmaco.gov.it/it/responsabili.
04.9 Overdose
Oral administration in mice, rats and dogs (male and female) of single doses up to 5000 mg / kg had no lethal effects.
No information is available on overdose in humans; Keraflox has been administered up to a dose of 1200 mg / day for 12 days in healthy volunteers showing overall good tolerability.
In the case of acute overdose, the stomach should be emptied by inducing vomiting or gastric lavage, the patient should be carefully followed and treated with symptomatic therapy.
05.0 PHARMACOLOGICAL PROPERTIES
05.1 Pharmacodynamic properties
Pharmacotherapeutic group: fluoroquinolones.
ATC code: J01MA17.
Prulifloxacin is an antibacterial belonging to the class of fluoroquinolones with a broad spectrum of action and high efficacy. After oral administration, prulifloxacin is absorbed from the gastrointestinal tract and immediately transformed into ulifloxacin, its active metabolite (see section 5.2).
Mechanism of action. Keraflox has been shown to be active in vitro, against a wide range of Gram-positive and Gram-negative strains. Prulifloxacin exerts its antibacterial action by selectively inhibiting DNA-gyrase, a vital enzyme found in bacteria, which is involved in DNA duplication, transcription and repair.
Resistance mechanism. The onset of antibiotic resistance to prulifloxacin (as well as to other fluoroquinolones) is generally due to spontaneous mutations in the bacterial DNA gyrase domain. In vitro, cross-resistance with other fluoroquinolones has been observed.
Due to the particular mechanisms of onset of resistance to fluoroquinolones, there is no cross-resistance between prulifloxacin and antibiotics of different classes, therefore Keraflox can be effective even in the presence of bacterial strains resistant to aminoglycosides, penicillins, cephalosporins and tetracyclines.
Intervals of inhibition. They have been defined on the basis of the NCCLS antibacterial activity data and the pharmacokinetic parameters of the product. The following inhibition ranges are suggested: Sensitive: MIC ≤ 1 mcg / ml, Intermediate: MIC> 1 to
Antibacterial spectrum. It should be considered that the prevalence of acquired resistance for selected species may vary geographically and with time, therefore local information on resistance is desirable, particularly when treating severe infections. If necessary, and if the local prevalence of resistance may make the drug usefulness questionable, it is advisable to seek expert advice.
The data reported in the table below indicate the antibacterial spectrum of prulifloxacin:
* Species showing a natural intermediate sensitivity.
Other information. In in vitro studies, the antibacterial action of prulifloxacin was characterized by better bacterial penetration and a more prolonged post-antibiotic effect than the reference fluoroquinolones.
05.2 Pharmacokinetic properties
a) General characteristics
Prulifloxacin is the prodrug of the active metabolite, ulifloxacin.
Absorption - In humans, prulifloxacin is rapidly absorbed (Tmax = approximately 1h) and transformed to ulifloxacin; after a single administration of 600 mg the mean peak plasma of ulifloxacin is 1.6 mcg / ml and the AUC is 7.3 mcg * h / ml. At steady-state, which is reached within 2 days of the initiation of once-daily dosing, the Cmax and AUC are 2.0 mcg / ml and 7.6 mcg * h / ml, respectively. .
Food delays and slightly reduces the peak plasma concentration of ulifloxacin, but does not change the AUC.
Distribution - In humans, the lung / plasma ratio of the mean concentration of Keraflox increases over time and, after 24 hours, the active metabolite ulifloxacin maintains mean tissue concentrations 5 times higher than those in plasma, confirming the results obtained in the animal. where the concentrations of ulifloxacin in the lung and kidney were higher than those in plasma (1.2 - 2.8 times and 3 - 8 times, respectively).
Similarly, human data on the tissue penetration of ulifloxacin into the paranasal sinuses showed, in terms of AUC, a tissue-to-plasma ratio of 3.0 in the ethmoid and 2.4 in the turbinates.
The protein binding in humans, assessed both in vitro that ex vivo, is approximately 50%, regardless of the drug concentration.
The low concentration of ulifloxacin found in the cerebrospinal fluid after i.v. in the dog and repeated administration p.o. in humans, it indicates that ulifloxacin hardly crosses the blood brain barrier.
Biotransformation - The metabolic profile of prulifloxacin in animals and humans is comparable. Animal studies have shown that the metabolism of prulifloxacin begins during intestinal absorption and is completed with its passage into the liver.
In addition to the transformation into ulifloxacin, other minor metabolites have been identified, such as the diol form and some derivatives such as glucuronide, oxo-derivative and ethylene-diamino derivatives, whose concentration and activity is negligible compared to the active principle.
No significant interactions with cytochrome P-450 isoenzymes were observed in in vitro studies, apart from a slight inhibition of CYP1A1 / 2 corresponding to a small decrease in theophylline clearance. Since methylxanthines, and in particular theophylline, constitute the main substrate for the CYP1A1 / 2 isoenzyme, the degree of interaction with other substrates of the isoenzyme (see warfarin) can be considered only lower.
Elimination - The half-life of the active metabolite, ulifloxacin, is approximately 10 hours after single and repeated steady-state administration in humans, while in animals (rats, dogs and monkeys) it varies between 2 and 12 hours.
Studies with the labeled product in humans have shown that elimination occurs mainly via the faecal route. After oral administration of 600 mg, the radioactivity recovered in urine and faeces amounts to approximately 95% in total. These results confirm what has been shown in previous studies carried out on animals (rats, dogs and monkeys).
The amount of ulifloxacin excreted in the urine is 16.7% of the administered dose on a molar basis and the renal clearance of ulifloxacin is approximately 170 ml / min.
Renal elimination of ulifloxacin occurs by glomerular filtration and by active secretion.
b) Characteristics in patients
The pharmacokinetic profile of prulifloxacin in the elderly has been shown to be similar to that in adults, with no change with age, and therefore no dosage adjustments are considered necessary in elderly patients.
In patients with mild or moderate renal insufficiency, after oral administration of Keraflox 600 mg, the mean plasma peak of ulifloxacin reaches values between 1.30 and 1.62 mcg / ml. The AUC values vary between 13.71 and 23.33 mcg * h / ml and the half-life between 12.3 and 32.4 hours. The renal clearance of ulifloxacin decreases compared to healthy volunteers depending on the degree of insufficiency.
05.3 Preclinical safety data
Repeated toxicity. In repeated dose toxicity studies, joint cartilage, kidney, gastrointestinal tract and liver were the main target organs. With doses up to 3 times higher than the therapeutic ones, no toxic effects on articular cartilages were observed (young dogs); with doses up to 6, 10 and 12 times higher than the therapeutic ones, no toxic effects were observed in the liver (dogs) and kidney (dogs and rats).
The drug does not prolong the QT interval in vivo and does not demonstrate inhibitory effects on delayed rectification potassium current (HERG) in vitro.
Reproductive toxicity. Reproductive toxicity studies did not show teratogenicity. Effects on fertility or on embryonic and fetal development were observed only in cases of maternal toxicity.
Mutagenicity. Standard genotoxicity assays have shown positive effects in some in vitro tests performed with prulifloxacin in mammalian cell cultures, but were negative in vivo and in bacteria.
These effects are believed to be associated with inhibition of topoisomerase II in the presence of high concentrations of prulifloxacin.
Carcinogenic potential. Prulifloxacin was not carcinogenic in a medium-term initiation-promotion model. Long-term carcinogenicity tests have not been performed.
Antigenicity. Prulifloxacin was found to have no antigenic effects.
Phototoxicity. Prulifloxacin induced phototoxic reactions, although in comparative studies in animals it has been shown to have a lower phototoxic activity than that of the other fluoroquinolones used (ofloxacin, enoxacin, pefloxacin, nalidixic acid and lomefloxacin). Many quinolones are also photarcomutagenic / photochemical. the possibility that prulifloxacin also has such effects cannot be excluded.
Nephrotoxicity. After repeated oral administration of 3000 mg / kg / day in rats, a dosage much higher than the therapeutic dose in humans, prulifloxacin caused crystalluria by precipitation of ulifloxacin.
Cardiotoxicity. Studies in dogs have shown that prulifloxacin does not cause noticeable changes in the electrocardiogram. In particular, no changes in QTc were observed either after single intravenous administration in the anesthetized dog, or after oral administration for 6 months in the conscious dog, at all. the doses administered. In vitro studies confirmed the absence of inhibitory effects on delayed potassium rectifying currents (HERG).
Joint toxicity. Prulifloxacin, similarly to other fluoroquinolones, caused arthropathy only in young animals.
Ocular toxicity. Oral doses of 26.4 or 58.2 mg / kg / day of prulifloxacin once daily for 52 weeks in monkeys did not cause treatment-related adverse effects on ocular function or morphology.
Rhabdomyolytic effect. Doses up to 10 mg / kg / day of ulifloxacin administered intravenously once daily for 14 consecutive days did not induce rhabdomyolysis in rabbits.
06.0 PHARMACEUTICAL INFORMATION
06.1 Excipients
Nucleus
lactose monohydrate,
microcrystalline cellulose,
croscarmellose sodium,
povidone,
anhydrous colloidal silica,
magnesium stearate
Coating
Hypromellose,
propylene glycol,
titanium dioxide (E171),
talc,
ferric oxide (E172).
06.2 Incompatibility
Not relevant
06.3 Period of validity
3 years
06.4 Special precautions for storage
Do not store above 30 ° C.
Store in the original packaging.
06.5 Nature of the immediate packaging and contents of the package
Cardboard box containing 1 blister of 1, 2, 5 film-coated tablets or 2 blisters of 5 film-coated tablets.
Blister in coupled material (Polyamide / aluminum / PVC) heat-sealed with covering material (aluminum / PVC).
Not all pack sizes may be marketed.
06.6 Instructions for use and handling
No special instructions.
Unused medicine and waste derived from this medicine must be disposed of in accordance with local regulations.
07.0 MARKETING AUTHORIZATION HOLDER
Crinos S.p.A. - Via Pavia, 6 - 20136 Milan
08.0 MARKETING AUTHORIZATION NUMBER
Box of 1 film-coated tablet of 600 mg A.I.C. 035680014
Box of 2 film-coated tablets of 600 mg A.I.C. 035680026
Box of 5 film-coated tablets of 600 mg A.I.C. 035680038
Box of 10 film-coated tablets of 600 mg A.I.C. 035680040
09.0 DATE OF FIRST AUTHORIZATION OR RENEWAL OF THE AUTHORIZATION
Date of first authorization: 21 June 2004
Authorization Renewal Date: 21 June 2009
10.0 DATE OF REVISION OF THE TEXT
February 2017