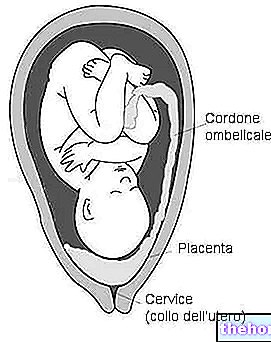
The tri test, or triple test if you prefer, is a small battery of biochemical tests, conducted on a sample of venous blood in order to quantify the risk of chromosomal abnormalities in the fetus. As the name suggests, the tri test is based on the analysis of the serum concentrations of three biochemical markers: alpha-fetoprotein, unconjugated estriol and human chorionic gonadotropin.

To carry out the tri-test, a simple maternal blood sample is sufficient, before which fasting is not necessary.
The interpretation of the results of the tri test - in relation to the age and other characteristics of the mother (weight, smoking, diabetes, etc.) - allows to quantify the risk of giving birth to a child with chromosomal abnormalities, such as the syndrome of Down. If this risk is greater than a certain threshold value (> 0.4%), an amniocentesis is then suggested, which allows to exclude or confirm the clinical suspicion. During this examination, in fact, it is possible to directly analyze the karyotype of the fetus (i.e. its chromosomal structure), thanks to the removal of fetal cells from the amniotic fluid. Unfortunately, as is known, amniocentesis is associated with a certain percentage of abortion risk (about 0.5%), connected to the invasiveness of the sampling technique.
Clinical significance
ALPHA FETOPROTEIN: in the presence of Down Syndrome it decreases by about 25-30%, while it increases significantly when there are defects of the neural tube or of the abdominal wall. Therefore, when the alpha-fetoprotein value is particularly high, the in-depth diagnostic examination is not amniocentesis but ultrasound.
NON-CONJUGATED ESTRIOL: like alphafetoprotein it decreases by about 25 - 30% in case of Down syndrome.
HUMAN CHORIONIC GONADOTROPIN: in pregnancies complicated by Down Syndrome it reaches values about two times higher than the norm.
The overall evaluation of these three markers in the blood means that the reliability of the Tri test reaches quite satisfactory values; in fact, it is estimated that the test recognizes about six to seven cases of Down syndrome out of ten. This percentage can further increase if the dosage of an additional marker, inhibin A, is added to the tri test (in this case, however, we no longer speak of tri but of quad test). In particular, the values of inhibin A are elevated when the fetus is affected by trisomy 21 (another name for Down syndrome).
The tritest cannot make a diagnosis, but it does express a probability.
The so-called duo test (or bi-test) supplemented by the evaluation of nuchal translucency is more reliable and can be performed earlier. This test, in fact, is able to identify up to 9 cases of fetuses with Down syndrome out of 10, with a risk of false positives equal to 5%; as anticipated, it is also performed between the 11th and 14th week of pregnancy, while the tri-test is performed later, between the fifteenth and twentieth week of gestation.
Finally, the so-called integrated test - which originates from the "integration of the triple test with the" bitest + nuchal translucency "combined exam - allows to raise the identification index up to 95 cases out of 100 (against 99 for amniocentesis ), with a very low percentage of false positives (about 1%).
Being a screening method, the "outcome of the examination" does not speak of positivity or negativity but - thanks to the "help of a software developed by the Fetal Medicine Foundation (London) and the evaluation of other parameters (maternal age, weight, smoking, diabetes, twin pregnancies, etc.) - expresses the risk in statistical percentage terms (for example 1 possible pathological case out of 1000 or one possible pathological case out of 100). be confused with the ability of the test to identify fetuses affected by Down syndrome. Rather, it means that among all those cases for which the "examination reports" attention, there is a certain risk that requires further investigations ", also includes a certain percentage of fetuses actually affected by Down syndrome (in this case 70-80%) . At the next amniocentic examination, therefore, the vast majority of fetuses will be confirmed free of any chromosomal anomaly; this is because pregnant women with a risk profile equal to or greater than 1: 250 are generally invited to perform this investigation (probability> 0.4 %).
The purpose of the tri test is only to identify women at greater risk, to whom it is possible to offer the possibility to carry out further investigations. the examination is therefore devoid of any diagnostic significance.
When undergoing a screening test for chromosomal abnormalities it must be borne in mind that:
- if the risk of disease is low, it does not mean that it is nil
- if the risk of disease is high this does not necessarily imply that the fetus is affected by a chromosomal abnormality; rather, it simply means that the risk is high enough to warrant a clarifying invasive examination (CVS, amniocentesis, or cordocentesis).
- therefore a pregnant woman who wishes to have absolute certainty about the absence of chromosomal abnormalities, and accepts the small risk associated with these diagnostic procedures, is directly directed to amniocentesis or CVS, bypassing the screening tests.
In light of the foregoing, in recent times the tri test - after having long been the most widespread among the various screening tests - has been supplanted by the "coupled" duo-test + nuchal translucency "; in any case, the tri test - supplemented with inhibin A dosage (quadruple test) - continues to be undertaken in cases where, for various reasons, the pregnant woman has not been able to undergo the aforementioned tests in good time.
Table I - Efficacy of screening tests for S. of Down
* the percentages may vary slightly in relation to the bibliography consulted
** The ability of the tri test to identify fetuses affected by neural tube defects is greater than that seen for chromosomal abnormalities, since it is around 90%.
LEGEND:
DR (detection rate): the proportion (in percent) of women with an affected fetus who test positive.
False positives: The proportion (in percent) of women with an unaffected fetus who test positive.
Note, for both parameters, the inferiority of the tri test compared to the other screening tests, both in terms of efficacy and quantification of the risk.







.jpg)


















