Generality
"Give me a fever and I will cure any disease": this statement, attributed to the Greek physician Hippocrates (400 BC), testifies how man has long understood the therapeutic potential of heat.
The first documentary evidence on the possible curative effect of high temperatures in the treatment of tumors dates back to 1866, when the German doctor Busch observed the complete remission of a sarcoma in the face of a patient after repeated bouts of high fever.

Today, due to the potential therapeutic benefits of this technique, hyperthermia is recognized as the fourth pillar of oncology.
What is oncological hyperthermia?
Oncological hyperthermia is a clinical treatment for the treatment of malignant tumors, which can be used alone or more frequently in combination with radiotherapy and chemotherapy treatments. Currently, in fact, this technique is not so much used as an alternative, but as an adjunct to other anticancer treatments. this association allows to obtain a reciprocal strengthening of the therapeutic efficacy. Furthermore, the association with hyperthermia allows to reduce the doses of chemotherapy and radiation, with a significant reduction of the side effects related to standard therapies.
Types of Hyperthermia
The therapeutic effect of hyperthermia for the treatment of tumors can be exploited using different approaches and technologies.
Cancer forms that have shown a good response to hyperthermia:
- Melanoma and other forms of skin cancer
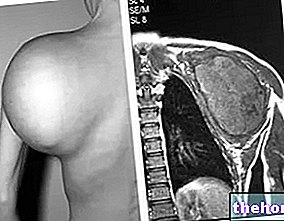
- Breast cancer
- Soft tissue sarcoma
- Bladder cancer
- Head and neck carcinomas
- Cervical and ovarian cancer
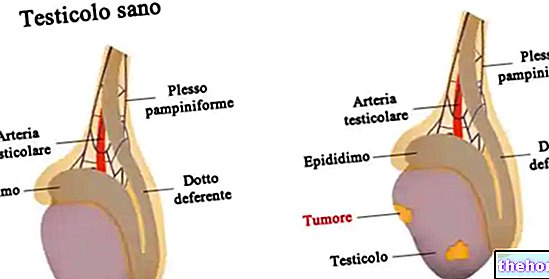
- Prostate Cancer
- Rectal cancer
- Axillary or chest wall carcinomas
The temperature and the duration of the exposure to heat are the two fundamental quantities to be calibrated in order to obtain the desired therapeutic result. However, in addition to the extent of the temperature reached and the time of application of the heat, it is very important to evaluate the source that generates the heating and its site of application. For example, micro-waves, radio frequencies, nanoparticles, ultrasounds, lasers can be used. etc., placed externally or internally to the body.
All these variables are chosen by the oncologist on the basis of the characteristics of the different clinical cases.
Results
In oncology, the chances of healing from a malignant tumor depend on many factors, such as the type and stage of the tumor, its size and location, the age and general health conditions of the patient.
Keeping all this in mind, several studies have shown that hyperthermia represents an excellent adjuvant to the classic treatment techniques for tumors, presenting few contraindications for patients.
For some types of tumors, associating radiotherapy (and / or chemotherapy) with hyperthermia, a 30-100% increase in complete remission rates and / or survival rates at 2 and 5 years was obtained, compared to to the use of radiotherapy alone (and / or chemotherapy). For some cancers, such as rectal cancer, the treatment results have proved even more encouraging (up to + 500% of the five-year survival rate).
Classic Hyperthermia 41-45 ° C
Classical oncological hyperthermia aims to warm cancer cells without damaging the surrounding healthy tissues.
- If the temperatures reached are between 41-43 ° C (mild hyperthermia) the main purpose is to increase the susceptibility of the tumor to radiotherapy and / or chemotherapy treatments.
- If the temperatures reached are between 43 and 46 ° C, the direct effect of heat on the killing of cancer cells becomes more important.
Depending on the case, the classic hyperthermia treatment lasts on average from 40 to 60 minutes and is repeated two to three times a week. More frequent treatments would in fact tend to induce thermoresistance (or thermotolerance if you prefer) in cancer cells, making them able to better withstand high temperatures.
Depending on the case, the heat source can have different sizes and can be located at different depths, in different organs or anatomical parts of the human body. For example, among the modern techniques of hyperthermia there is also the possibility of implanting microwave antennas directly in the subcutis.
How does it work
DIRECT DAMAGE TO TUMOR CELLS
The efficacy of oncological hyperthermia is based on the chaotic angiogenesis of tumor tissues. Basically, the tumor microenvironment almost always presents a chaotic and disorganized vascular scaffold; as a result, large tumor areas (especially the central mass) receive insufficient amounts of blood and oxygen. Due to these alterations of the blood vessels, the neoplastic mass is unable to dissipate heat like normal tissues; in other words, tumors tend to suffer much more heat than healthy tissues, because some of their areas receive little blood (which acts as a real coolant); for the same reason, these areas are already suffering from the scarcity of oxygen and nutrients and the abundance of waste products (hyperacidification).
The heat administered by hyperthermia causes damage to the plasma membrane, the cell skeleton and the nucleus; if the extent and duration of the hyperthermia are sufficient, this damage leads directly to the death of the tumor cell. Direct damage becomes significant at temperatures> 43 ° C: indirect damage, which we will see shortly, is instead typical of the so-called "mild hyperthermia" (42-43 ° C).
INDIRECT DAMAGE: ADJUVANT HYPERTHERMIA
Our body reacts to the local temperature rise by increasing blood flow to the affected area. In this way the greater quantities of circulating blood "absorb" heat, preserving the tissues from thermal damage. This response also occurs at the tumor level, so - within the limits of the peculiar vascular disorganization - tumor cells subjected to a slight increase in temperature receive greater quantities of blood and oxygen:
- antitumor drugs may be present in the blood, which thanks to the vasodilation induced by hyperthermia are able to more easily reach the less vascularized neoplastic areas; the action of these drugs could also be facilitated by cellular (increased permeability of the plasma membrane) and enzymatic alterations (protein denaturation) induced by heat.
When temperatures in the tumor mass exceed 43 ° C, on the other hand, a decrease in tumor blood flow is recorded, with consequent "trapping" of the drug molecules.
The advantages of the hyperthermia-chemotherapy combination have been confirmed by several studies. Antitumor drugs such as Melphalan, Bleomycin, Adriamycin, Mitomycin C, Nitrosuree, Cisplatin are more effective when administered during hyperthermia. In this regard, however, it should be emphasized that not all known chemotherapy drugs find an enhancement of their effectiveness if used in a hyperthermic environment. - The greater supply of oxygen to the tumor tissue amplifies the effects of radiotherapy, which are mainly based on DNA damage induced by reactive oxygen species (free radicals) generated by radiation. As seen for chemotherapy, the activity of radiotherapy is facilitated also from the neoplastic cellular compromise linked to the damage previously inflicted by the hyperthermia.
The reciprocal completion and reinforcement of action between hyperthermia and radiotherapy derives from the fact that:- the damage induced by hyperthermia is greater in areas with low vascularization (which cannot effectively dissipate heat), such as the hypoxic central nucleus of the neoplastic nodule;
- the damage induced by radiotherapy is instead greater in areas with high vascularization (richer in oxygen), such as the peripheral mantle areas of the tumor nodule;
- the two treatments perform their maximum damaging efficacy on the tumor in different phases of the cell cycle, being complementary also in this sense.
The maximum therapeutic gain seems to be obtained by practicing the hyperthermic treatment within one or two hours after the radiotherapy session. As for thermochemotherapy, however, the two treatments can also be performed simultaneously.
Oncological hyperthermia can contribute to the reduction of the tumor mass in view of a surgical removal operation. There are also benefits in terms of analgesic effect (reduction of pain triggered by the compression of the tissues by the neoplastic mass).
Other forms of hyperthermia
TOTAL-BODY HYPERTHERMIA
As the name suggests, this form of hyperthermia provides for the heating of the whole organism. The aim, in this case, is not to directly destroy the tumor mass, but to determine its indirect remission by means of an immune system strengthening. Quest "The latter, in fact, has an intrinsic ability to destroy cancer cells, and this ability increases enormously in conditions of high body temperature.
The purpose of total-body hyperthermia is to induce an artificial fever, simulating a febrile attack around 39-41 ° C. In this regard, thermal or water-covered chambers can be used.
The use of the total-body is mostly confined to the experimental setting for the treatment of diffuse metastases. The technique requires close monitoring of the patient to avoid damage from hyperthermia, which can also be very serious. It is also an adjuvant therapy, from therefore to be used in association with other anticancer therapies.
INTERSTIZIAL HYPERTHERMIA
As seen for brachytherapy - in which small radioactive sources are implanted in the target tissue - "interstitial hyperthermia involves the implantation of devices capable of generating a" local hyperthermia. In this regard, antennas are used that heat thanks to the supply of microwave.
INFUSIONAL HYPERTHERMIA and PERFUSION HYPERTHERMIA
Intraperitoneal infusional hyperthermia is based on the use of peritoneal washes with medicated solutions at high temperatures. It is used in cases of peritoneal neoplasms that are difficult to treat, such as peritoneal mesothelioma and stomach cancer. Other hyperthermia techniques are based on the same principle, which involve the infusion of therapeutic solutions heated into other cavities, such as the pleural or bladder cavity.
In perfusion hyperthermia, extracorporeal circulation is used, with heating of part of the blood and re-introduction of the same with the addition of chemotherapeutic drugs, in order to obtain high drug concentrations in the perfused tissue.
ABLATIVE HYPERTHERMIA
In this case the temperatures are much higher (50-100 ° C), but they are applied only for a few minutes. Such temperatures are capable of producing an immediate and total necrosis of the treated tissues. The heat is generated by the application of an alternating electric current through electrodes or by the use of laser or electromagnetic radiation, applied directly on the tumor mass (invasive treatment). The greatest difficulty lies in preserving the healthy tissues surrounding the tumor.
Although this technique exploits the therapeutic effect of heat, for the mechanism of action it goes beyond what is the traditional concept of hyperthermia.
NEW DEVELOPMENTS IN THE "FIELD OF HYPERTHERMIA"
The science of hyperthermia is constantly evolving to develop increasingly selective treatments in order to destroy cancer cells without damaging healthy ones.
The most recent developments concern the non-invasive thermometry with the use of magnetic resonance scanners (to evaluate the temperature in the different tumor areas), the hyperthermia magneto fluid and the use of thermosensitive liposomes. The latter are drugs enclosed in lipid vesicles, stable at normal body temperatures but capable of releasing their contents at temperatures of about 40-43 ° C; these drugs therefore represent the ideal combination with regional hyperthermia treatments.
Limits
Understanding the mechanisms of action of hyperthermia and the consequent potential benefits in the treatment of tumors could lead to excessive enthusiasm of the reader towards this type of treatment.
Although it is supported by fair evidence of efficacy, the application of hyperthermia in oncology retains some critical issues. First of all, in clinical practice, there may be contraindications or limits that make the intervention impracticable; some techniques, for example, provide for real more or less invasive surgical interventions; others are still confined mostly to the experimental environment. It is also necessary to overcome limits. technicians connected to the emission of heat, the depth of penetration, the homogeneity of the thermal fields and the need for a correct thermal dosage to avoid damage to healthy tissues. In this regard, further studies and technological developments are desirable to develop effective protocols and standardized to be adopted in different clinical situations.