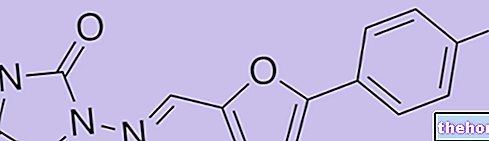
TALAVIR ® is a drug based on Valaciclovir
THERAPEUTIC GROUP: Antivirals for systemic use

Indications TALAVIR ® Valaciclovir
TALAVIR ® is indicated in the treatment of infections caused by Herpes Simplex type 1 and 2, Varicella Zooster Virus and in the prophylaxis of the infection and related Cytomegalovirus disease linked to transplant procedures.
Mechanism of action TALAVIR ® Valaciclovir
TALAVIR ® is a drug based on Valaciclovir, a molecule consisting of L-Valine and aciclovir, therefore considered a prodrug of Aciclovir, characterized by decidedly more favorable pharmacokinetic properties, including its bioavailability.
Taken orally it is absorbed and subsequently converted into Aciclovir in the intestine and liver to be subsequently distributed among the various tissues where it can carry out its therapeutic action.
More precisely, once the plasma membranes of the host cells are permeated, it is converted into Aciclovir triphosphate in two successive steps supported respectively by viral enzymes such as viral thymidino kinase and finally by cellular kinases.
The newly formed Aciclovir triphosphate, thanks to the analogy with purine nuclohexides, interposes itself to the nascent DNA chain by blocking the activity of the viral DNA polymerase enzyme and thus inhibiting the potential mechanisms of viral replication.
However, the aforementioned activity can be limited by the establishment of resistance mechanisms such as to reduce the efficacy of Aciclovir by:
- The reduction of the binding affinity between Aciclovir and viral DNA polymerase;
- The absence of the viral thymidine kinase initiator enzyme;
- The reduction of the binding affinity between Aciclovir and viral thymidine kinase.
Studies carried out and clinical efficacy
VALACICLOVIR IN THE TREATMENT OF EBV-INDUCED LYMPHOPROLIFERATIVE DISORDERS
Clin Lymphoma Myeloma Leuk. 2012 Dec 20. pii: S2152-265000246-7.
Phase I Clinical Trial of Valaciclovir and Standard of Care Cyclophosphamide in Children With Endemic Burkitt Lymphoma in Malawi.
Olson D, Gulley ML, Tang W, Wokocha C, Mechanic O, Hosseinipour M, Gold SH, Nguluwe N, Mwansambo C, Shores C.
Very interesting clinical trial that demonstrates how the use of Valaciclovir can be effective in determining an improvement of the symptoms associated with EBV dependent pathologies such as for example for lymphoproliferative disorders.
VALACICLOVIR IN THE PROPHYLAXIS OF CMV INFECTIONS IN TRANSPLANTED PATIENTS
Nephrol Dial Transplant. 2012 Dec 14.
Clinical outcome with low-dose valaciclovir in high-risk renal transplant recipients: a 10-year experience.
Sund F, Tufveson G, Döhler B, Opelz G, Eriksson BM.
Considering that CMV infections still remain one of the most important risks associated with transplant procedures, the use of low doses of Valaciclovir for 90 days after transplantation could represent a particularly important preventive strategy.
VALACICLOVIR AND RETINITE
BMC Ophthalmol. 2012 Sep 5; 12: 48.
Valaciclovir in the treatment of acute retinal necrosis.
Taylor SR, Hamilton R, Hooper CY, Joshi L, Morarji J, Gupta N, Lightman SL.
Very recent study that demonstrates how the oral administration of Valaciclovir can be effective in the treatment of acute retinal necrosis, guaranteeing a complete resolution of the disease.
Method of use and dosage
TALAVIR ®
Coated tablets of 500 mg - 1000 mg of Valaciclovir.
The definition of the dosage and the therapeutic scheme envisaged for TALAVIR ® must necessarily be defined by a doctor competent in the treatment of infectious diseases, taking into account:
- The physiopathological conditions of the patient;
- Of his immunological picture;
- His age and the possible presence of contraindications to the use of the drug;
- The severity of the clinical picture;
- From therapeutic goals.
Warnings TALAVIR ® Valaciclovir
The use of TALAVIR ® must necessarily be preceded by a careful medical examination aimed at evaluating the prescribing appropriateness and the possible presence of contraindications to the use of the drug.
Particular caution should in fact be reserved for patients suffering from liver and kidney diseases, in which, given the impaired ability to excrete the drug, side effects and adverse reactions may occur with a higher incidence.
In order to reduce the spread of the virus, it would be advisable, together with drug therapy, to apply a series of hygiene rules to control the human-to-human transmission of the pathogen.
Prolonged use of TALAVIR ®, in addition to increasing the incidence of potential side effects, could favor the onset and spread of viral strains resistant to drug therapy and therefore responsible for particularly serious clinical pictures.
PREGNANCY AND BREASTFEEDING
Given the ability of Aciclovir, pharmacologically active form of Valaciclovir, to cross the blood-placental barrier and the mammary filter, fortunately only rarely reaching clinically relevant concentrations, it would be appropriate to extend the aforementioned contraindications to the use of the drug also to pregnancy and the subsequent period. breastfeeding.
Should the needs require the use of TALAVIR ®, it is the task of the gynecologist to establish the usefulness of the therapy on the basis of the cost / benefit ratio.
Interactions
Although the pharmacological interactions between Aciclovir and other active ingredients are quite rare and clinically of little relevance, it would however be advisable to pay particular caution to the simultaneous intake of drugs capable of altering renal function, thus determining the accumulation of the active ingredient and the possible onset of side effects.
Contraindications TALAVIR ® Valaciclovir
The use of TALAVIR ® is contraindicated in patients hypersensitive to Aciclovir or to structurally related active ingredients rather than to excipients present in the drug.
Undesirable Effects - Side Effects
The use of TALAVIR ® could cause the onset of diarrhea, abdominal pain, nausea, headache and vomiting.
Much rarer are clinically relevant adverse reactions characterized by renal and hepatic pathologies, hypersensitivity reactions and neurological disorders.
Note
TALAVIR ® is a drug subject to mandatory medical prescription.
The information on TALAVIR ® Valaciclovir published on this page may be out of date or incomplete. For a correct use of this information, see the Disclaimer and useful information page.