What is Repaglinide Krka?
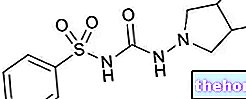
Repaglinide Krka is a medicine containing the active substance repaglinide, available as tablets (white: 0.5 mg; yellow: 1 mg; pink: 2 mg).
Repaglinide Krka is a 'generic medicine'. This means that it is similar to a 'reference medicine' already authorized in the European Union (EU) called NovoNorm. For more information on generic medicines, please see the questions and answers by clicking here.
What is Repaglinide Krka used for?
Repaglinide Krka is used in patients with type 2 diabetes (non-insulin-dependent diabetes). It is used in conjunction with specific diet and exercise regimes to reduce blood glucose (sugar) levels in patients whose hyperglycaemia (high blood glucose levels) can no longer be controlled through diet, weight loss and exercise. .
The medicine can only be obtained with a prescription.
How is Repaglinide Krka used?
Repaglinide Krka is taken before meals, normally up to 15 minutes before each meal. The dose should be adjusted to achieve the best possible control. The treating physician should regularly measure the patient's blood glucose level to find the lowest effective dose. Repaglinide Krka may also be indicated for type 2 diabetics who are usually well controlled on a diet, but who are going through a passing phase in which the body cannot regulate the level of glucose in the blood.
The recommended starting dose is 0.5 mg. This dose may be increased after one or two weeks.
If patients switch to Repaglinide Krka while they are already using another antidiabetic, the recommended starting dose is 1 mg.
Repaglinide Krka is not recommended for use in patients below 18 years of age in the absence of information on the safety and efficacy of the product for this age group.
How does Repaglinide Krka work?
Type 2 diabetes is a disease in which the pancreas does not make enough insulin to control the level of glucose in the blood or where the body is unable to use insulin effectively. Repaglinide Krka helps the pancreas to produce more insulin during meals and is used to control type 2 diabetes.
How has Repaglinide Krka been studied?
Since Repaglinide Krka is a generic medicine, the studies have been limited to evidence designed to show that the medicine is bioequivalent to the reference medicine, NovoNorm. Two medicines are bioequivalent when they produce the same levels of the active substance in the body.
What are the risks and benefits associated with Repaglinide Krka?
Since Repaglinide Krka is a generic medicine and is bioequivalent to the reference medicine, the benefits and risks are assumed to be the same as the reference medicine.
Why has Repaglinide Krka been approved?
The Committee for Medicinal Products for Human Use (CHMP) concluded that, in accordance with the requirements of EU legislation, Repaglinide Krka has been shown to have comparable quality and to be bioequivalent to NovoNorm. It is the view of the CHMP that, as in the case of NovoNorm, the benefits outweigh the identified risks. The Committee recommended that the marketing authorization be given for Repaglinide Krka.
Other information about Repaglinide Krka:
On November 4, 2009, the European Commission granted Krka, d.d., Novo mesto a "marketing authorization" for Repaglinide Krka, valid throughout the European Union.
For the full version of the Repaglinide Krka EPAR, click here.
The full EPAR version of the reference medicine can also be found on the Agency's website.
Last update of this summary: 11-2009.
The information on Repaglinide Krka published on this page may be out of date or incomplete. For a correct use of this information, see the Disclaimer and useful information page.