What is Plegridy and what is it used for?
Plegridy is a medicine that contains the active substance peginterferon beta-1a. It is indicated for the treatment of multiple sclerosis (MS), a disease in which an "inflammation destroys the protective sheath that lines the nerve fibers. It is particularly indicated in adult patients with a form of multiple sclerosis known as" relapsing-remitting "(when that is, the patient suffers from exacerbations of symptoms (relapses) followed by periods of recovery (remissions).
How is Plegridy used - peginterferon beta-1a?
Plegridy can only be obtained with a prescription and treatment should be started under the supervision of a doctor who has experience in treating MS. Plegridy is available as a solution for injection in pre-filled pens that contain 63, 94 or 125 micrograms of peginterferon beta-1a. Treatment should start with a dose of 63 micrograms, followed by a dose of 94 micrograms two weeks apart, then continued with a dose of 125 micrograms every two weeks. Plegridy is given by subcutaneous injection into the abdomen, arm or thigh. The patient can inject the medicine himself after receiving the appropriate instructions. See the package leaflet for further information.
How does Plegridy work - peginterferon beta-1a?
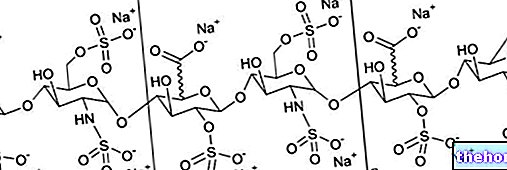
In multiple sclerosis, the body's immune system does not function properly and attacks some parts of the central nervous system (made up of the brain and spinal cord), causing inflammation that damages the nerve sheaths. The mechanism of action of Plegridy in MS is not yet fully known but the active ingredient contained in the medicine, peginterferon beta 1-a, appears to reduce the activity of the immune system (the body's natural defenses) and prevent relapse of the SM Interferon beta 1-a is a form of protein that is naturally produced by the body. The interferon in Plegridy is produced by a method known as 'recombinant DNA technology': it is made by cells that have received a gene (DNA) that allows them to produce human interferon. The interferon is then 'pegylated' (ie bound to a chemical called 'polyethylene glycol'). This treatment reduces the rate at which the substance is cleared from the body and allows the medicine to be given less often.
What benefit has Plegridy - peginterferon beta-1a shown during the studies?
As part of a two-year main study involving 1,516 patients, Plegridy was shown to reduce the relapse rate in patients with relapsing-remitting MS. During the first year, patients were treated with Plegridy or with placebo (a dummy treatment) every two to four weeks; in the second year, all patients were treated with Plegridy every two to four weeks. The main measure of effectiveness was the number of relapses the patients reported over a 1 year period. , although the study also looked at other parameters including how quickly disability progressed. In the first year, patients treated with Plegridy every two to four weeks reported on average fewer relapses than patients treated with placebo: 0.26 and 0, respectively. 29 relapses compared to 0.40. Disability progression decreased in subjects treated with Plegridy every two weeks, while the data appears less clear in patients. i treated every four weeks. In the second year of therapy, Plegridy continued to produce benefits. The study was extended for a further two years to examine the long-term safety and efficacy of Plegridy, and the data from this second phase available at the time of authorization were consistent with the results of the main study.
What is the risk associated with Plegridy - peginterferon beta-1a?
The most common side effects with Plegridy (which may affect more than 1 in 10 people) are headache, myalgia (body aches), arthralgia (joint pain), flu-like symptoms, pyrexia (fever), chills, asthenia (weakness) and erythema (redness of the skin), pain or itching at the injection site. Treatment with Plegridy should not be started during pregnancy. In addition, Plegridy should not be used in patients with severe depression or with suicidal thoughts. For the complete list. of all side effects and limitations reported with Plegridy, see the package leaflet.
Why has Plegridy - Peginterferon beta-1a been approved?
The Agency's Committee for Medicinal Products for Human Use (CHMP) decided that Plegridy's benefits are greater than its risks and recommended that it be approved for use in the EU. The CHMP considered that Plegridy administered every two weeks has shown to induce an approximately 30% reduction in the number of relapses in relapsing-remitting MS patients compared to placebo, a result that is comparable to that seen with other MS medicines containing non-pegylated interferon beta, and therefore is considered clinically relevant. In addition, the CHMP is of the opinion that Plegridy offers patients greater benefit when given every two weeks than the less frequent doses tested in the study. When Plegridy was given every four weeks, its positive effect was less and not it was possible to identify a group of patients in whom this less frequent dosage could be considered ade guato. Regarding the safety profile, the most common adverse events observed during treatment with Plegridy are considered manageable and, in general, are consistent with the events observed with the use of non-pegylated interferon medicines.
What measures are being taken to ensure the safe and effective use of Plegridy - peginterferon beta-1a?
A risk management plan has been developed to ensure that Plegridy is used as safely as possible. Based on this plan, safety information has been added to the summary of product characteristics and package leaflet for Plegridy, including the appropriate precautions to be followed by healthcare professionals and patients. Further information can be found in the summary of the risk management plan.
More information about Plegridy - peginterferon beta-1a
On 18 July 2014, the European Commission granted a "Marketing Authorization" for Plegridy, valid throughout the European Union. For more information on Plegridy therapy, read the package leaflet (included with the EPAR) or consult your doctor or the pharmacist. Last update of this summary: 07-2014.
The information on Plegridy - peginterferon beta-1a published on this page may be out of date or incomplete. For a correct use of this information, see the Disclaimer and useful information page.