What is Pemetrexed medac and what is it used for?
Pemetrexed medac is an anticancer medicine used in the treatment of two types of lung cancer:
- malignant pleural mesothelioma (a cancer of the tissue lining the lungs, usually caused by "exposure to" asbestos), in which the medicine is used in combination with cisplatin in patients who have not previously received chemotherapy and whose cancer cannot be removed by surgical route;
- advanced non-small cell lung cancer, of the type known as 'non-squamous', in which the medicine is used in combination with cisplatin in patients not previously treated, or on its own in patients who have previously received anticancer treatment . It can also be used as maintenance therapy in patients who have undergone platinum-based chemotherapy.
Pemetrexed medac is a 'generic medicine'. This means that Pemetrexed medac is similar to a 'reference medicine' already authorized in the European Union (EU) called Alimta. For more information on generic medicines, see the questions and answers by clicking here.
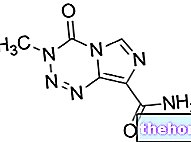
Pemetrexed medac contains the active substance pemetrexed.
How is Pemetrexed medac used?
Pemetrexed medac is available as a powder that is made up into a solution for infusion (drip) into a vein. The medicine can only be obtained with a prescription and should only be given under the supervision of a doctor experienced in the use of chemotherapy.
The recommended dose is 500 mg per square meter of body surface area (calculated based on the patient's height and weight), and is given as a 10-minute infusion once every three weeks. To reduce side effects, patients should take a corticosteroid (a type of medicine that reduces inflammation) and folic acid (a type of vitamin) and receive vitamin B12 injections during treatment with Pemetrexed medac. When Pemetrexed medac is given with cisplatin, patients should also take an 'antiemetic' drug (to prevent vomiting) and fluids (to prevent dehydration) before or after the cisplatin dose.
Treatment should be postponed or stopped, or the dose reduced, in patients with abnormal blood counts or who have certain other side effects. For more information, see the Summary of Product Characteristics (included in the EPAR).
How does Pemetrexed medac work?
The active substance in Pemetrexed medac is pemetrexed, a cytotoxic medicine (a medicine that kills actively dividing cells, such as cancer cells) that belongs to the group of 'antimetabolites'. In the body, pemetrexed is converted into an active form that blocks the activity of the enzymes involved in the production of "nucleotides" (the building blocks of DNA and RNA, the genetic material of cells).Consequently, the active form of pemetrexed slows down the formation of DNA and RNA and prevents the division and multiplication of cells. The conversion of pemetrexed to its active form occurs more rapidly in cancer cells than in normal cells; for this reason, in cancer cells there are higher concentrations of the active form of the drug and a longer duration of action. The division of cancer cells is therefore reduced, while normal cells are only partially affected.
How has Pemetrexed medac been studied?
The company presented data on pemetrexed from the scientific literature. No further studies were needed because Pemetrexed medac is a generic medicine that is given by infusion and contains the same active substance as the reference medicine, Alimta.
What are the benefits and risks of Pemetrexed medac?
Because Pemetrexed medac is a generic medicine, its benefits and risks are taken as being the same as the reference medicine's.
Why has Pemetrexed medac been approved?
The Agency's Committee for Medicinal Products for Human Use (CHMP) concluded that, in accordance with EU requirements, Pemetrexed medac has been shown to be comparable to Alimta. Therefore, the CHMP considered that, as in the case of Alimta, the benefits outweigh the identified risks and recommended that Pemetrexed medac be approved for use in the EU.
What measures are being taken to ensure the safe and effective use of Pemetrexed medac?
A risk management plan has been developed to ensure that Pemetrexed medac is used as safely as possible. Based on this plan, safety information has been added to the summary of product characteristics and package leaflet for Pemetrexed medac, including the appropriate precautions to be followed by healthcare professionals and patients. Further information can be found in the summary of the risk management plan.
More information about Pemetrexed medac
For more information about Pemetrexed medac therapy, read the package leaflet (included with the EPAR) or contact your doctor or pharmacist.
The full version of the EPAR for the reference medicine can also be consulted on the Agency's website.
The information on Pemetrexed medac published on this page may be out of date or incomplete. For a correct use of this information, see the Disclaimer and useful information page.