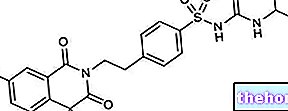
MINIDIAB ® a drug based on Glipizide.
THERAPEUTIC GROUP: Oral hypoglycemic agents - Sulfonylureas

Indications MINIDIAB ® - Glipizide
MINIDIAB ® is used as a pharmacological aid in type II diabetic patients, when diet and exercise are not sufficient to guarantee good glycemic control.
Mechanism of action MINIDIAB ® - Glipizide
Glipizide, active ingredient of MINIDIAB ®, is a second generation sulphonylurea useful in the management of diabetic patient hyperglycemia.
Taken orally, it is readily absorbed in the intestine, guaranteeing a sensitive hypoglycemic effect, only after half an hour from oral intake.
Its therapeutic action is due to the ability to reach the pancreatic beta cell, bind to the ATP-dependent potassium channel by inhibiting it, and facilitate membrane depolarization with a consequent increase in intracellular calcium concentrations, useful for ensuring the fusion of insulin-containing vesicles. with the cell membrane and the consequent release of this hormone.
Several studies also seem to agree on the protective effect of glipizide, useful in reducing the thrombotic risk, safeguarding the integrity of endothelial cells, seriously compromised in patients with type II diabetes.
Studies carried out and clinical efficacy
1. GLIPIZIDE AND BODY COMPOSITION
Diabetes Care. 2006 Mar; 29: 510-4.
Effects of pioglitazone versus glipizide on body fat distribution, body water content, and hemodynamics in type 2 diabetes.
Basu A, Jensen MD, McCann F, Mukhopadhyay D, Joyner MJ, Rizza RA.
The treatment with glipizide, contrary to what has been observed for other oral hypoglycemic drugs, did not have positive results on the body composition, leaving the percentage of adipose tissue concentrated in the abdominal area almost unchanged without guaranteeing particular improvements in the values of systolic and diastolic pressure.
2. GLIPIZIDE AND METFORMIN
Diabetes Res Clin Pract. 2005 May; 68: 167-75.
Effect on glycemic control of the addition of 2.5 mg glipizide GITS to metformin in patients with T2DM.
Feinglos M, Dailey G, Cefalu W, Osei K, Tayek J, Canovatchel W, Chaiken R, Kourides I.
This study has shown that the addition of 2.5 mg of glipizide to metformin, in patients with type II diabetes not responsive to monotherapy with metformin, can significantly increase the therapeutic efficacy of the drug, ensuring good glycemic control and a significant decrease in the concentrations of glycosylated hemoglobin.
3.GLIBIZIDE: THE IMPORTANCE OF CORRECT DOSAGE
Acta Diabetol. 2010 May 29.
Effect of sulfonylurea dose escalation on hemoglobin A1c in Veterans Affairs patients with type 2 diabetes.
Hurren KM, Bartley EP, O "Neill JL, Ronis DL.
The formulation of the correct dosage is one of the most influencing factors on the safety and efficacy of the therapeutic protocol with oral hypoglycemic agents. reduction of the percentages of glycosylated hemoglobin by up to 1%.
Method of use and dosage
MINIDIAB ® Glipizide 5 mg tablets:
hypoglycemic treatment should begin with the lowest effective dose of ½ tablet daily taken before main meals.
Based on the glycemic control obtained from the initial dose for approximately two weeks of treatment, the appropriate dosage should be set never exceeding 4 tablets per day.
A further adjustment of the hypoglycemic therapy may be required during the work or in the case of concomitant use of other antidiabetic drugs.
MINIDIAB ® - Glipizide warnings
MINIDIAB ® should be used in type II diabetic patients as a pharmacological aid to control blood sugar levels in combination with an adequate lifestyle and a balanced diet.
Before and during therapy, the blood glucose concentration should be periodically assessed, in order to establish and possibly adapt the dosage of the drug during the course of work, to avoid the onset of hyper and hypoglycemic episodes and to ensure good metabolic control.
Medical supervision is also of fundamental importance for those patients with reduced liver and kidney function, for whom the hypoglycemic effects of the therapy could be partially accentuated.
It should be remembered that an inadequate dosage of MINIDIAB ® could increase the risk of hypoglycemia, anticipated by some warning signs, making the use of machinery and driving vehicles dangerous.
PREGNANCY AND BREASTFEEDING
Glipizide, like other sulfonylureas and other oral hypoglycemic drugs, is contraindicated both during the gestation period and in the subsequent lactation phase.
In these moments, people generally opt for the use of more studied hypoglycemic drugs with a standardized therapeutic effect such as insulin.
Interactions
Derivatives of dicumarol and dicumarol, monoamine oxidase inhibitors, sulfonamides, phenylbutazone, chloramphenicol, cyclophosphamide, probenecid, phenirmidol and salicylates may increase the hypoglycemic effect of MINIDIAB ® increasing the risk of hypoglycemia in the treated patient.
On the contrary, adrenaline, corticosteroids, oral contraceptives and thiazide diuretics can reduce the therapeutic efficacy of glipizide, preventing the drug from controlling glucose metabolism.
Interactions with even serious consequences may also occur following the simultaneous use of glipizide and alcohol or warfarin.
Contraindications MINIDIAB ® - Glipizide
MINIDIAB ® is contraindicated in patients suffering from diabetes mellitus of the first type, from severe liver and kidney dysfunctions, from precoma and diabetic coma, diabetic keto acidosis, in case of hypersensitivity to the active ingredient or to one of its excipients and during pregnancy and " feeding time
Undesirable Effects - Side Effects
Hypoglycemic therapy with second generation sulfonylureas such as glipizide contained in MINIDIAB ® proved to be much safer and well tolerated than older sulfonylureas.
In fact, the cases of hypoglycemia, although present, have been significantly reduced, remaining confined to the most susceptible patients such as the elderly, alcoholics, patients with reduced liver and kidney function and patients treated with excessive doses of the drug.
Gastrointestinal disturbances, changes in the haematological picture, hypersensitivity reactions and decreased liver function were observed only in rare cases and promptly recovered upon discontinuation of therapy.
Note
MINIDIAB ® can only be sold under strict medical prescription
The information on MINIDIAB ® - Glipizide published on this page may be out of date or incomplete. For a correct use of this information, see the Disclaimer and useful information page.