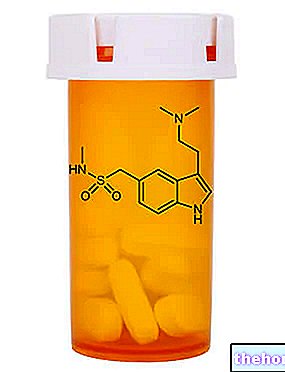
What is Lyrica?
Lyrica is a medicine that contains the active substance pregabalin. It is available in capsule form (white: 25 mg, 50 mg and 150 mg; white and orange: 75 mg, 225 mg and 300 mg; orange: 100 mg; light orange: 200 mg).
What is Lyrica used for?
Lyrica is used to treat adults with the following conditions:
- neuropathic pain (pain caused by damage to the nervous system). Lyrica can be used to treat peripheral neuropathic pain, for example in diabetic patients or patients with herpes zoster (St. Anthony's focus), and central neuropathic pain, which affects for example patients who have suffered a spinal cord injury;
- epilepsy. Lyrica is given as an add-on to ongoing therapy in patients with partial seizures (seizures that begin in a "specific area of the brain) that cannot be controlled with ongoing therapy;
- generalized anxiety disorder (chronic anxiety or nervousness about issues related to everyday life).
The medicine can only be obtained with a prescription.
How is Lyrica used?
The recommended starting dose of Lyrica is 150 mg per day, divided into two or three doses. After three to seven days, the dose can be increased to 300 mg per day. Doses can be increased up to more than twice as much until the most effective dose is reached. The maximum daily dose is 600 mg per day. Stopping treatment with Lyrica should also be done gradually, over at least one week.
The capsules should be swallowed whole with water, with or without food. In patients with kidney problems the dosage is lower.
How does Lyrica work?
The active substance in Lyrica, pregabalin, is similar in structure to the organism's "neurotransmitter" gamma-amino butyric acid (GABA), but has very different biological effects. Neurotransmitters are chemicals that allow nerve cells to communicate with each other. The precise modes of action of pregabalin are not fully known, but pregabalin is thought to affect the way calcium enters nerve cells. This reduces the activity of some nerve cells in the brain and spinal cord, with a consequent reduction in the release of other neurotransmitters that are involved in pain, epilepsy and anxiety.
How has Lyrica been studied?
Lyrica was compared with placebo (a dummy treatment) in 22 studies:
- ten studies have been conducted for neuropathic pain, involving more than 3,000 patients with peripheral neuropathic pain. About half of the patients had diabetic neuropathy, the other half had pain from Sant 'Antonio's fire. Another study was performed in 137 patients with central neuropathic pain due to spinal cord injury. The studies lasted up to 12 weeks and the effectiveness of Lyrica was measured using a standard pain questionnaire;
- for epilepsy, three studies were conducted involving a total of over 1 000 patients. The change in the number of seizures after a period of 11-12 weeks was the main measure of effectiveness;
- Eight studies involving over 3,000 patients were performed for generalized anxiety disorder. Effectiveness was measured using a standard anxiety questionnaire after four to eight weeks.
What benefit has Lyrica shown during the studies?
In neuropathic pain studies, Lyrica was more effective than placebo in reducing pain. In the peripheral neuropathic pain studies, 35% of patients treated with Lyrica had a 50% or more decrease in pain score compared to 18% of patients treated with placebo. In central neuropathic pain studies, 22% of patients treated with Lyrica had a 50% or more decrease in pain score compared to 8% of patients treated with placebo.
In epilepsy studies, Lyrica reduced the number of seizures: approximately 45% of patients taking Lyrica 600 mg had a 50% or more reduction in seizures and approximately 35% reduction in subjects receiving 300 mg daily. day, compared with approximately 10% of patients treated with placebo.
In the studies in generalized anxiety disorder, Lyrica was more effective than placebo: 52% of the patients taking Lyrica experienced a 50% or greater improvement compared to 38% of the patients taking placebo.
What are the risks associated with Lyrica?
The most common side effects seen with Lyrica (seen in more than 1 in 10 patients) are dizziness and sleepiness. For the full list of side effects reported with Lyrica, see the package leaflet.
Lyrica must not be used in people who may be hypersensitive (allergic) to pregabalin or any of the other substances.
Why has Lyrica been approved?
The Committee for Medicinal Products for Human Use (CHMP) decided that Lyrica's benefits are greater than its risks in the treatment of peripheral and central neuropathic pain in adults, as adjunctive therapy in adults with partial seizures with or without secondary generalization and in treatment of generalized anxiety disorder in adults. The committee recommended that Lyrica be given a marketing authorization.
Other information about Lyrica:
On 6 July 2004, the European Commission granted Pfizer Limited a "Marketing Authorization" for Lyrica, valid throughout the European Union. The "Marketing Authorization" was renewed on 6 July 2009.
For the full version of Lyrica's EPAR, click here.
Last update of this summary: 07-2009.
The information on Lyrica - pregabalin published on this page may be out of date or incomplete. For a correct use of this information, see the Disclaimer and useful information page.