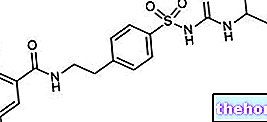
JANUMET ® a drug based on Sitagliptin and Metformin
THERAPEUTIC GROUP: Associated oral hypoglycemic agents

Indications JANUMET ® - Sitagliptin + Metformin
JANUMET ® is used in the treatment of type II diabetes, when metformin alone, even at maximum doses, has not been able to guarantee good glycemic control.
In patients with severe metabolic decompensation, JANUMET ® may be included in triple therapy with sulfonylurea.
Mechanism of action JANUMET ® - Sitagliptin + Metformin
JANUMET ® represents an oral hypoglycemic drug, obtained from the combination of active ingredients belonging to the category of DPP-4 inhibitors and biguanides.
The therapeutic effect, which is carried out through an integrated control of glucose metabolism, is mediated by sitagliptin, capable of increasing the availability of incretins (reducing the activation of DPP-4, involved in irreversible hydrolysis), hormones responsible for sensitization of the pancreatic beta cell useful for ensuring the release of insulin, and of metformin, capable of acting on various insulin sensitive tissues by increasing the uptake of blood glucose, and in the liver by inhibiting the process of gluconeogenesis and glycogenolysis.
From a metabolic point of view, therefore, the hypoglycemic effect is supported on the one hand by the increased secretion of insulin and on the other by the reduced endogenous production of glucose.
From a pharmacokinetic point of view, the intake of JANUMET ® maintains the characteristics of the two active ingredients taken individually almost unchanged.
Studies carried out and clinical efficacy
1. THE EFFECTIVENESS OF THE SITAGLIPTIN / METFORMIN COMBINED THERAPY
Drugs. 2011 Feb 12; 71: 349-61.
Sitagliptin / metformin fixed-dose combination: in patients with type 2 diabetes mellitus.
Chwieduk CM.
Studies have shown that the combination of metformin and sitagliptin can ensure good glycemic control in those patients with type II diabetes mellitus, for whom metformin alone was ineffective. In these cases, a further reduction in blood glucose was observed. "glycosylated hemoglobin, excellent basic and postprandial glycemic control and a reduced risk of hypoglycemia.
2. SITAGLIPTIN / METFORMIN, BEYOND CLINICAL PRACTICE
Diabetes Res Clin Pract. 2011 Feb 21
Effect of sitagliptin plus metformin on β-cell function, islet integrity and islet gene expression in Zucker diabetic fatty rats.
Han SJ, Choi SE, Kang Y, Jung JG, Yi SA, Kim HJ, Lee KW, Kim DJ.
The important therapeutic successes that the association between metformin and sitagliptin has achieved in clinical practice, seem essentially associated with the single therapeutic effects of the two active ingredients. However, in vitro studies have shown how this combination can act synergistically on the beta cell, preserving function and integrity. probably by activating anti-apoptotic genes involved in cell survival.
3. SITAGLIPTIN / METFORMIN IN ONE FORMULATION
Clin Drug Investig. 2010; 30: 855-66.
Bioequivalence of sitagliptin / metformin fixed-dose combination tablets and concomitant administration of sitagliptin and metformin in healthy adult subjects: a randomized, open-label, crossover study.
Migoya EM, Miller JL, Gutierrez M, Zheng W, Johnson-Levonas AO, Liu Q, Matthews CZ, Wagner JA, Gottesdiener KM.
Pharmacokinetic studies and clinical trials of various kinds have concluded that the therapeutic effect observed following the intake of JANUMET, may be fully comparable to that obtained with the separate but simultaneous administration of metformin and sitagliptin, with the same frequency of side effects.
Method of use and dosage
JANUMET ® 50/850 or 50/1000 mg tablets of sitagliptin and metformin:
therapy with JANUMET ® should start from the lowest administrable dose, and then eventually correct or validate the aforementioned dosage, based on the metabolic finding observed in terms of blood glucose concentration.
The maximum dose should not exceed that of 100 mg of sitagliptin, possibly taken in two different administrations concurrently with meals to reduce gastrointestinal side effects.
In the event that triple therapy with sulfonylureas should be used, dosages lower than those foreseen could also be used.
In any case, it is up to your doctor to evaluate and formulate the appropriate dosage.
Warnings JANUMET ® - Sitagliptin + Metformin
It is very important to ensure correct glycemic control, that treatment with JANUMET ® is accompanied by periodic monitoring of glycemic levels and the concomitant application of non-pharmacological measures such as physical activity and a balanced diet.
It is also necessary to monitor renal function, to avoid that the accumulation of metformin induces in the patient a state of metabolic acidosis, which is potentially lethal.
Treatment with JANUMET ® should be suspended in cases where the use of iodinated contrast media or drugs or procedures that compromise renal function is required.
Hypoglycemic conditions, potentially verifiable following incorrect dosages, administration of active ingredients or other causes, could reduce the patient's perceptive and reactive abilities, making the use of machinery and driving cars dangerous.
PREGNANCY AND BREASTFEEDING
The use of JANUMET ® in pregnancy is strongly contraindicated, due to the presence of studies showing an increased risk of congenital malformations in newborns delivered to women treated with metformin.
On the contrary, the experimentation with sitagliptin has not produced statistically significant results at the moment.
Furthermore, the possible secretion of these active ingredients in breast milk exposes the infant to the potential risk of hypoglycemia, so much so as to extend the contraindication also to the lactation period.
Interactions
Also in this case the drug interactions observed for JANUMET ® are to be attributed in a distinct way to the presence of the two active ingredients.
Consequently, while sitagliptin is poorly reactive, with pharmacokinetic changes observed following the administration of digoxin and cyclosporine, metformin may respond negatively to the concomitant administration of glucorticoids, beta agonists, cimetidine, and diuretics.
Several studies have also evaluated the presence of other possible interactions in vitro, which however assume modest clinical relevance in vivo.
It is also useful to remember that the concomitant administration of rifampicin or iodinated contrast media could alter normal renal function, increasing the body's exposure to these hypoglycemic drugs.
Contraindications JANUMET ® - Sitagliptin + Metformin
JANUMET ® is contraindicated in case of hypersensitivity to the active ingredients or excipients, type I diabetes, diabetic keto acidosis, severe changes in liver and kidney function, dehydration, shock, acute pathologies, heart and respiratory failure, shock, alcoholism, pregnancy and breastfeeding. .
Undesirable Effects - Side Effects
The side effects described following therapy with JANUMET ® are essentially attributable to those observed during monotherapy with sitagliptin and metformin.
Especially the initial phase of therapy may be accompanied by gastrointestinal disturbances such as nausea, vomiting, constipation or diarrhea, upper respiratory tract infections and headache and dizziness.
Clinically more significant adverse reactions were observed only rarely, with the appearance of hypersensitivity reactions such as angioedema, rash and urticaria, changes in the haematological picture and in the electrocardiographic trace, for which it was necessary to suspend the therapy.
Hypoglycaemic conditions, on the other hand, were found above all in the course of triple therapy with sulfonylureas.
Note
JANUMET ® can only be sold under strict medical prescription
The information on JANUMET ® - Sitagliptin + Metformin published on this page may be out of date or incomplete. For a correct use of this information, see the Disclaimer and useful information page.







.jpg)


















