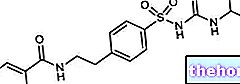
GLURENOR ® a drug based on Gliquidone.
THERAPEUTIC GROUP: Oral hypoglycemic agents - Sulfonylureas

Indications GLURENOR ® - Gliquidone
GLURENOR ® is indicated in the treatment of type II diabetes mellitus.
Mechanism of action GLURENOR ® - Gliquidone
GLURENOR ® is an oral hypoglycemic drug, whose efficacy is closely related to the active ingredient gliquidone, belonging to the pharmaceutical category of sulfonylureas.
As such, this active ingredient, taken orally, is rapidly absorbed in the intestine, reaching its maximum plasma concentration after about 60 - 90 minutes and persisting for about 3 - 4 hours, at the end of which it is metabolized in the liver. a series of inactive metabolites eliminated mainly via the bile.
Its hypoglycemic efficacy is evidently due to the ability to bind and inhibit the ATP-dependent potassium channel expressed by pancreatic beta cells, facilitating the depolarization of the plasma membrane, useful for ensuring the release of insulin.
Several studies seem to agree on the ability of gliquidone to reproduce a post-prandial insulin secretion that is very close to the physiological one and therefore able to guarantee excellent glycemic control.
Studies carried out and clinical efficacy
1. FLUID AND MANAGEMENT OF TYPE II DIABETIC PATIENT
Drugs R D.2006; 7: 331-7.
Gliquidone contributes to improvement of type 2 diabetes mellitus management: a review of pharmacokinetic and clinical trial data.
Malaisse WJ.
Clinical experience has shown that most patients with type II diabetes tend to develop diabetic nephropathy with reduced renal function over time. One of the most important limitations of pharmacological therapies in this case is represented by the way of disposal of the inactive metabolites of the drug, often urinary. Gliquidone is very well suited for the treatment of diabetic patients suffering from nephropathy, given the scarce involvement of the kidney in the excretion of the drug.
2. GLURENOR AND PROTECTION FROM OXIDATIVE STRESS
Drug Toxicol Chem. 2005; 28: 483-97.
Protective effects of glurenorm (gliquidone) treatment on the liver damage of experimental diabetes.
Yanardag R, - Sacan Ozsoy O, H Orak, Ozgey Y.
The development of oxygen free radicals and reactive species represents one of the most frequent complications of diabetic pathology, which over time tends to be associated with macro and microangiopathies and organ damage. Experimental studies have shown how gliquidone can reduce oxidative stress on the liver, supporting the activity of glutathione and counteracting the toxic effect of oxidizing agents.
3. THE LIQUID AND POST TRANSPLANTATION DIABETES
Clin Nephrol. 2008 Jul; 70: 26-32.
Gliquidone therapy of new-onset diabetes mellitus after kidney transplantation.
Tuerk TR, Bandur S, Nuernberger J, Kribben A, Mann K, Philipp T, Heemann U, Viklicky O, Witzke O.
The onset of diabetes after kidney transplantation represents one of the most important complications, often associated with increased morbidity and mortality. In this case, the treatment with gliquidone was found to be safe and effective in controlling glycemic concentrations, reducing the renal workload.
Method of use and dosage
GLURENOR ® Gliquidone 30 mg tablets:
the formulation of the correct dosage should be established by the doctor after a "careful evaluation of the patient's physio-pathological picture, of the glycaemia levels and of the responsiveness to therapy.
In principle, the dose should range between 30 and 120 mg of gliquidone per day.
Warnings GLURENOR ® - Gliquidone
In any case, therapy with GLURENOR ® should be preceded and accompanied by a correct lifestyle and a healthy diet. Periodic monitoring of glycemic levels throughout the therapeutic plan is essential in evaluating the efficacy of the therapy and possibly in the formulation of new, more suitable dosages.
The administration of GLURENOR ® should take place with particular care and under strict medical supervision in patients with impaired hepatic and renal function.
The risk of hypoglycemia, which occurs as a result of inappropriate therapy or incorrect eating habits, could make driving cars and using machines dangerous, therefore it is important that the patient is instructed by his doctor on the recognition of possible warning signs of this condition.
PREGNANCY AND BREASTFEEDING
Gestational diabetes, a frequent condition in pregnancy and potentially dangerous for the health of the unborn child, is generally treated with hypoglycemic drugs with the most characterized mechanism of action and the highest safety profile such as insulin.
Consequently, in these conditions the use of GLURENOR ® is contraindicated.
Interactions
It is important to consider that the metabolic effect of sulfonylureas can be significantly modulated by the concomitant intake of other active ingredients, making the therapeutic effect of the drug unpredictable.
More precisely, it is known that dicumarol and dicumarol derivatives, monoamine oxidase inhibitors, sulfonamides, phenylbutazone, chloramphenicol, cyclophosphamide, probenecid, phenirmidol and salicylates can enhance the therapeutic effect of GLURENOR ® increasing the risk of hypoglycemia, while adrenaline, corticosteroids and thiazide diuretics can reduce the therapeutic efficacy of gliquidone, making glycemic control unstable.
Interactions with even serious consequences may also occur following the simultaneous use of sulfonylureas and alcohol or warfarin.
Contraindications GLURENOR ® - Gliquidone
GLURENOR ® contraindicated in patients suffering from diabetes mellitus of the first type, from severe liver and kidney dysfunctions, from precoma and diabetic coma, diabetic keto acidosis, in case of hypersensitivity to the active substance or to one of its excipients and during pregnancy and lactation
Undesirable Effects - Side Effects
Although therapy with sulfonylureas, and in this case with GLURENOR ® is very well tolerated and without clinically relevant side effects, it is possible to describe episodes of hypoglycemia in debilitated, elderly, liver disease patients or following excessive doses. of gliquidone.
In this case the symptoms characterized by headache, decreased alertness, mental confusion, delirium, convulsions, bradycardia, somnolence and loss of consciousness could be avoided by the administration of sugars.
Rarely, sulfonylurea therapy has been associated with gastrointestinal disturbances, changes in the blood picture and skin hypersensitivity reactions.
Note
GLURENOR ® can only be sold under strict medical prescription.
The information on GLURENOR ® - Gliquidone published on this page may be out of date or incomplete. For a correct use of this information, see the Disclaimer and useful information page.