GALVUS ® a drug based on Vidagliptin
THERAPEUTIC GROUP: Oral hypoglycemic agents - DPP-4 inhibitors

Indications GALVUS ® - Vidagliptin
GALVUS ® is a drug useful in the treatment of hyperglycemia of type 2 diabetes mellitus, in combination with metformin, sulfonylureas and PPAR gamma agonists, in case of insufficient therapeutic response observed following monotherapy.
Mechanism of action GALVUS ® - Vidagliptin
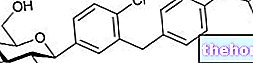
Vidagliptin, the active ingredient of GALVUS ®, is a very useful molecule to ensure correct glycemic control, thanks to its indirect ability to improve the glucose response of the beta cell and therefore the subsequent production and secretion of insulin.
More precisely, this active principle acts by selectively inhibiting the DPP-4 enzyme, involved in the degradation of incretins (GLP1 and GIP), hormones produced at the level of the gastro-intestinal tract, and useful in sensitizing the beta cell to increased glucose concentrations. to ensure a correct response in terms of insulin secretion, while modulating the production and secretion of glucagon.
From the metabolic point of view, this complex mechanism of action translates into a reduction in the post-prandial hyperglycemic peak and fasting glycaemia, through a greater availability of insulin and a reduced production of endogenous glucose.
From a pharmacokinetic point of view, on the other hand, GALVUS ® fully belongs to oral hypoglycemic agents, from the moment in which, taken orally, it is absorbed in the intestine, reaching the maximum plasma concentration in about 2 hours, to be subsequently eliminated in the form of metabolites. inactive mainly via the kidney.
Studies carried out and clinical efficacy
1. VIDAGLIPTIN AND METFORMIN
Diabet Med. 2010 Mar; 27: 318-26.
A comparison of efficacy and safety of vildagliptin and gliclazide in combination with metformin in patients with Type 2 diabetes inadequately controlled with metformin alone: a 52-week, randomized study.
Filozof C, Gautier JF.
It is known that vidagliptin can be used concomitantly with metformin in the treatment of diabetic patients unresponsive to metformin alone. In this study, in fact, the combined therapy, prolonged for 52 weeks, guaranteed a further lowering of glycosylated hemoglobin since " 7% to 6.5% significantly reducing cases of hypoglycemia and weight gain.
2. VIDAGLIPTIN AND GLIMEPIRIS IN THE TREATMENT OF THE DIABETIC PATIENT
Diabetes Res Clin Pract. 2010 Sep; 89: 216-23.
Efficacy and tolerability of vildagliptin as an add-on to glimepiride in Japanese patients with Type 2 diabetes mellitus.
Kikuchi M, Haneda M, Koya D, Tobe K, Onishi Y, Couturier A, Mimori N, Inaba Y, Goodman M.
Treatment of the diabetic patient with vidagliptin and glimepiride ensured a significant reduction in glycosylated hemoglobin, and excellent control of fasting and postprandial glycaemia, much higher than monotherapies. The side effects recorded were modest with only two cases of hypoglycemia.
3. VIDAGLIPTIN AND ACUTE PANCREATITIS
Endocr Pract. 2011 Feb 16: 1-6.
Acute Pancreatitis in a Patient Receiving Vildagliptin.
Girgis CM, Champion BL.
Case report that focuses on the possibility of vidagliptin to determine the onset of acute pancreatitis. Although it is currently the first case, the severity of this pathology obliges the various international bodies to monitor the possible side effects of incretin-based therapy.
Method of use and dosage
GALVUS ® vidagliptin 50 mg tablets:
the recommended dosage is two tablets a day of GALVUS ® taken regardless of meals.
However, it is always necessary to remember that the correct dosage should be formulated by one's doctor based not only on the physio-pathological condition of the patient, but also on the possible presence of combined therapies with other hypoglycemic drugs.
Warnings GALVUS ® - Vidagliptin
It is important that drug therapy for the treatment of type II diabetic patients is accompanied by non-pharmacological measures such as healthy nutrition and a correct lifestyle.
The modest clinical trial that characterizes this type of drug did not allow to better characterize the activity of GALVUS ® in patients suffering from cardiac and hepatic diseases, therefore, medical supervision would be ideal in these cases.
Monitoring of blood glucose and transaminases as well as renal function is therefore useful for the therapy to maintain the correct balance, reducing the risk of potential side effects.
GALVUS ® contains lactose, therefore the administration in patients with lactase enzyme deficiency, lactose intolerance or glucose / galactose absorption deficiency, could be accompanied by the concomitant presence of side effects concentrated especially at the gastrointestinal level.
The risk of hypoglycaemia could make the use of machinery and driving vehicles dangerous.
PREGNANCY AND BREASTFEEDING
The contraindication to the use of GALVUS ® during pregnancy and lactation derives essentially from the absence of studies that test the safety profile of the drug on the health of the fetus when taken during pregnancy, and from the availability of other well-characterized drugs on the market. , useful for the management of gestational diabetes.
Interactions
The various experiments and the various pharmacokinetic tests have demonstrated the low degree of interaction between vidagliptin and other active ingredients, probably also justified by the poor hepatic metabolism this active ingredient undergoes.
However, it should be remembered that the concomitant intake of other oral hypoglycemic drugs could enhance the therapeutic effects of GALVUS ® thus increasing the risk of hypoglycemia.
Contraindications GALVUS ® - Vidagliptin
GALVUS ® is contraindicated in patients hypersensitive to the active substance or to one of its excipients, in patients with type I diabetes, diabetic ketoacidosis, hepatic and renal insufficiency and during pregnancy and lactation.
Undesirable Effects - Side Effects
The various clinical trials carried out have described, following the intake of vidagliptin, modest and transient side effects such as not to require discontinuation of therapy.
Among the side effects, the most observed were angioedema, nausea, weight gain, headache and asthenia, while the reactions affecting the liver and the skin region following hypersensitivity to the active ingredient were rarer but clinically more significant.
However, it is important to remember that some of these effects have been observed in combination therapies.
Note
GALVUS ® can only be sold under strict medical prescription
The information on GALVUS ® - Vidagliptin published on this page may be out of date or incomplete. For a correct use of this information, see the Disclaimer and useful information page.