Water requirement
Water is an essential component of our organism; in adults it represents over 70% of the total mass (in children it is even higher) and its systemic deficiency can compromise well-being, health and (at worst) survival. of the person. The risk increases significantly in old age, when the body is more prone to dehydration and the brain transmits / perceives only a few "thirst" signals.
It goes without saying that the water must be:
- Drunk in a manner quantitatively sufficient (about 1milliliter each calorie introduced with the diet - 1ml / 1kcal - therefore two liters per day in the case of a 2000 Kcal diet)
- Distributed equally during the day.

Water and digestibility of the meal
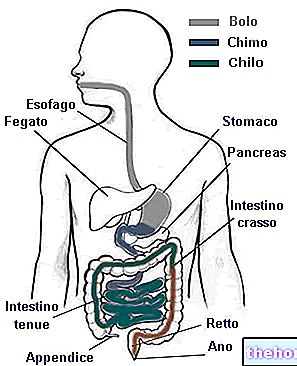
Digestion is an active process that provides for the simplification of nutritional polymers, aimed at allowing their absorption in the intestine.
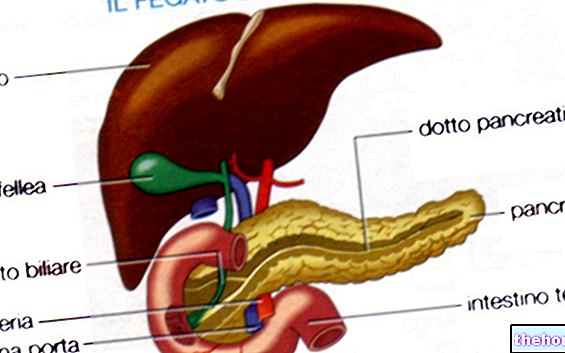
Digestion is organized in different chemical-physical stages and starts from the mouth, reaches the stomach and ends in the intestine. The mechanical phases are chewing and kneading (oral cavity), mixing (stomach), advancement and segmentation (intestine). chemical phases determine the secretion of glands and various exocrine glandular tissues; they occur in the mouth (saliva with salivary amylase), in the stomach (gastric juices with pepsinogen, hydrochloric acid [Hcl-] and pepsin), in the duodenum (in which, through the choledochus, biliary and pancreatic juices are introduced [numerous proteo-, lipo- and glycolytic enzymes]) and on the mucosa of the small intestine (enzymes of the brush border of the enterocytes).
What is often overlooked is that digestion, in order to take place in an optimal way, requires the secretion / dilution of enzymes PROPORTIONAL to the "consistency" of the meal. In short:
- Saliva, gastric, biliary and pancreatic juices require WATER to be produced and secreted.
- The less water is present in the food bolus / chyme, the more the "organism is obliged to secrete" from its own pocket ".
It follows that, in an excessively "dry" meal, the water required to give the right moisture to the bolus / chyme (and promote its digestibility) is greater than that required by a well-hydrated meal. "Excessive dilution of the meal could compromise digestion due to the excessive dispersion of gastric juices and enzymes.
NB. The absorption / reabsorption of water occurs mainly between the stomach and the duodenum BUT it ends definitively in the large intestine through fecal dehydration (recovery of the water secreted with digestive juices).
Promote digestion
In general, digestion occurs in an optimal way by consuming one or two glasses of water (depending on the capacity) during the meal. This parameter varies considerably depending on the presence or absence of "soupy" foods (which in themselves contribute to dilute the food bolus), fresh and well hydrated foods (vegetables and fruit) and the quantity of dry or dehydrated foods (breadsticks, crackers, fries in bags, popcorn, salted meats, dried fruit, etc.).
In addition to the excessive quantity, protein share, cooking level of the meal and any individual "deficiencies" (or pathologies), many other chemical and physical factors contribute to determining the POOR efficacy and the time dilation useful for digestion; among these: concentration of table salt (NaCl), food pH, chewing, food temperature etc.
On the other hand, there are many "tricks" to be used occasionally to facilitate the digestion of an excessive or heavy meal; the choice of one or the other depends above all on the food introduced and on the physiological condition of the subject. problem consists in the reduced ability to secrete hydrochloric acid, following a sensibly protein meal, it may be advisable:
- Take hot water (35-38 ° C) with the addition of juice, or better, of lemon zest
- Take one "alcoholic unit, if habitually consumed
- Drink cola-like drinks
- Take coffee drinks, if usually consumed
- Chewing gum
NB. Under similar conditions, the presence of table salt and spices in the meal can be conducive to the secretion of HCl.
On the other hand, if the meal is excessively protein and if consequently (in most cases), an overproduction of hydrochloric acid occurs, the bolus / food chyme (after protein denaturation) requires a "conversion" of the pH to enter the duodenum from acid to basic by secretion of bicarbonates. In this case, after the meal it would be useful:
- Take water at room temperature with bicarbonate, citrate (citrosodine) or magnesium hydroxide (magnesia)
- Avoid the 5 points mentioned above.
Is there a "water that promotes digestion?"
According to what has been said so far, water is an essential element of the meal, useful (and sometimes fundamental) for the success of digestion, but if introduced in excess, it can cause excessive dilution of digestive juices, prolonging digestion times.
By now, everyone knows that waters are not all the same; they differ above all in the content and origin of the salts they contain. If they come from spontaneous sources they are called minerals and their purity is NOT obtained with chemical-physical purification; otherwise, like tap water, (although it also contains salts), being manipulated by man, it cannot be defined as a "mineral".
Some waters have characteristics potentially useful for digestion; the dissolved parts (in this case "active ingredients") useful for this purpose are:
- Bicarbonates (HCO3)
- Sulphates (SO4)
The bicarbonates, as anticipated, participate in the reduction of gastric pH by counteracting "acidity" and determining the reduction of the time spent in the stomach. The use of water with bicarbonates is indicated for all subjects who tend to suffer from gastric acidity and / or who consume very large and protein meals.
NB. The presence of bicarbonates in the water does NOT justify neglect or excesses in the management of meals; the abuse of salty, spicy, alcoholic, coffee, acidic and caffeinated drinks, etc. it cannot be counteracted by water containing bicarbonates.
The sulphates, on the other hand, have a "pro-digestive action thanks to their ability to stimulate enzymatic synthesis in the liver and pancreas; in this way, the composition of the digestive juices (if slightly deficient) can be compensated for by promoting digestion.
In conclusion, the most suitable "mineral" waters to counteract gastric hyperacidity and promote digestion are those rich in bicarbonates and sulphates; however, to be clear, it should be borne in mind that the quantity of dissolved salts (while representing a desirable characteristic ) in itself is not sufficient to cancel the unwanted effects of an excessively large meal.




.jpg)


















