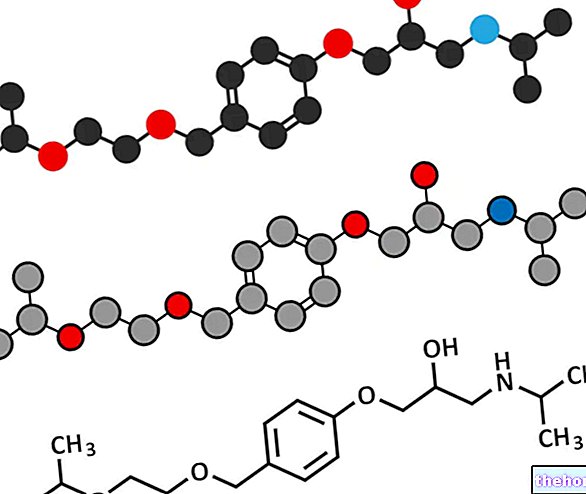
Active ingredients: Clopidogrel
Plavix 75 mg film-coated tablets
Plavix package inserts are available for pack sizes:- Plavix 75 mg film-coated tablets
- Plavix 300 mg film-coated tablets
Why is Plavix used? What is it for?
Plavix contains clopidogrel and belongs to a group of medicines called antiplatelet agents. Platelets are microscopic elements of the blood that clump together during blood clotting. By preventing this clumping, antiplatelet medicines reduce the chance of blood clots forming (a phenomenon called thrombosis).
Plavix is taken by adults to prevent blood clots (thrombi) forming in hardened blood vessels (arteries), a process known as atherothrombosis, which can cause atherothrombotic events (such as stroke, heart attack, or death). Plavix has been prescribed to you to help prevent blood clots and to reduce the risk of these serious events because:
- you have a condition known as hardening of the arteries (also called atherosclerosis), e
- you have previously had a heart attack, stroke or a condition known as peripheral arterial disease, or
- you have previously suffered from severe chest pain known as 'unstable angina' or 'myocardial infarction' (heart attack). To treat this condition, your doctor may have placed a stent in your blocked or narrowed artery to restore blood flow. Your doctor may also have prescribed acetylsalicylic acid (a substance found in many medicines used to relieve pain and reduce fever, such as also to prevent blood clotting),
- you have an irregular heartbeat, a condition called 'atrial fibrillation', and cannot take medicines known as 'oral anticoagulants' (vitamin K antagonists) which prevent new clots from forming and developing existing ones. You will have been told that "oral anticoagulants" are more effective than acetylsalicylic acid or the combined use of Plavix and acetylsalicylic acid in treating this condition. If you cannot take "oral anticoagulants" and are not at increased risk of bleeding, your doctor may have prescribed Plavix plus acetylsalicylic acid.
Contraindications When Plavix should not be used
Do not take Plavix
- If you are allergic (hypersensitive) to clopidogrel or any of the other ingredients of this medicine (listed in section 6).
- If you have active bleeding, such as a "stomach ulcer or bleeding in an area of the brain.
- If you have severe liver disease.
If you think any of these apply to you, or if you are in any doubt at all, consult your doctor before using Plavix.
Precautions for use What you need to know before taking Plavix
If any of the situations mentioned below occur, tell your doctor before taking Plavix:
- if you have a risk of bleeding such as: - a medical condition that puts you at risk for internal bleeding (such as a "stomach ulcer) - a blood disorder that makes you prone to internal bleeding (bleeding inside any tissue, organ or body joint) - a recent serious injury - recent surgery (including dental surgery) - surgery (including dental surgery) scheduled for the next 7 days
- if you have had a clot in an "artery of the brain (ischemic stroke) that has occurred within the past 7 days
- if you have kidney or liver disease
- if you have ever had an allergy or reaction to any medicine used to treat your disease
While you are taking Plavix:
- You should tell your doctor if you need to have surgery (including dental surgery)
- You should tell your doctor right away if you develop a medical condition (also known as Thrombotic Thrombocytopenic Purpura or PTT) which includes fever and bruises under the skin that appear as red dots, with or without unexplained extreme fatigue, confusion, yellowing of the skin or eyes ( jaundice) (see section 4 "Possible side effects")
- If you cut or injure yourself, it may take longer than usual for the bleeding to stop. This is due to the way the medicine works as it prevents blood clots from forming. For minor cuts and injuries, such as cutting yourself or shaving, this usually isn't a problem. However, if you are concerned by your bleeding, you should contact your doctor straightaway (see section 4 'Possible side effects')
- Your doctor may order blood tests
Children and adolescents
Do not give this medicine to children as it is not effective.
Interactions Which drugs or foods may change the effect of Plavix
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines, even those obtained without a prescription.
Some medicines may influence the use of Plavix or vice versa.
You must tell your doctor precisely if you are taking:
- oral anticoagulants, medicines used to reduce blood clotting,
- a non-steroidal anti-inflammatory medicine, generally used to treat painful and / or inflammatory conditions of muscles or joints,
- heparin or any other injectable drug used to reduce blood clotting,
- omeprazole, esomeprazole or cimetidine, medicines used to treat stomach problems,
- fluconazole, voriconazole, ciprofloxacin, or chloramphenicol, medicines used to treat bacterial or fungal infections,
- carbamazepine, or oxcarbazepine, medicines used to treat some forms of epilepsy,
- ticlopidine, other antiplatelet agents,
- a selective serotonin reuptake inhibitor (including but not limited to fluoxetine or fluvoxamine), medicines normally used to treat depression,
- moclobemide, a medicine used to treat depression.
If you have had severe chest pain (unstable angina or heart attack), you may have been prescribed Plavix in combination with acetylsalicylic acid, a substance found in many medicines used to relieve pain and reduce fever. Occasional use of acetylsalicylic acid (no more than 1,000 mg in 24 hours) should not generally cause problems, but prolonged use in other circumstances should be discussed with your doctor.
Plavix with food and drink
Plavix can be taken with or without food.
Warnings It is important to know that:
Pregnancy and breastfeeding
It is preferable not to take this medicine during pregnancy.
If you are pregnant or suspect that you are pregnant, you should tell your doctor or pharmacist before taking Plavix. If you become pregnant while taking Plavix, consult your doctor immediately, as it is recommended not to take Plavix during pregnancy.
You should not breastfeed while taking this medicine. If you are breastfeeding or planning to breastfeed, consult your doctor before taking this medicine.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
Plavix is unlikely to affect the ability to drive and use machines.
Plavix contains lactose
If you have been told by your doctor that you have an "intolerance to some sugars (eg lactose), consult your doctor before taking this medicine.
Plavix contains hydrogenated castor oil
This can cause stomach upset or diarrhea.
Dose, Method and Time of Administration How to use Plavix: Posology
Always take this medicine exactly as your doctor or pharmacist has told you.
If in doubt, consult your doctor or pharmacist. If you have had severe chest pain (unstable angina or heart attack), your doctor may give you 300 mg of Plavix (1 tablet of 300 mg or 4 tablets of 75 mg) once at the start of treatment. Thereafter, the dose recommended is one 75 mg tablet of Plavix per day, to be taken orally with or without food, and at the same time each day.
Plavix must be taken for as long as the doctor deems it necessary.
Overdose What to do if you have taken too much Plavix
If you take more Plavix than you should
Contact your doctor or the emergency room of the nearest hospital because of the risk of increased bleeding.
If you forget to take Plavix
If you forget to take a dose, but remember within 12 hours of your usual time, take one tablet straight away and then take the next one at the usual time.
If it has been more than 12 hours, simply take your normal dose at the usual time. Do not take a double dose to make up for a forgotten tablet.
For packs of 7, 14, 28 and 84 tablets, you can check the day the last Plavix tablet was taken by checking the calendar printed on the blister.
If you stop taking Plavix
Do not stop treatment unless your doctor tells you to. Before stopping it, contact your doctor or pharmacist.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
Side Effects What are the side effects of Plavix
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Contact your doctor immediately if:
- fever, signs of infection or severe weakness. These effects may be due to a rare decrease in some blood cells
- signs of liver problems such as yellowing of the skin and / or eyes (jaundice), with or without bleeding which appears under the skin as red pinpoint dots, and / or confusion (see section 2 "Warnings and precautions")
- swelling in the mouth or skin disorders such as rash, itching, blistering of the skin. These can be signs of an allergic reaction.
The most common side effect reported with Plavix is bleeding. Bleeding can manifest itself as bleeding in the stomach or intestines, bruising, bruising (unusual bleeding or bruising under the skin), nosebleeds, blood in the urine. In a few cases, bleeding in the eye, intracranial, lungs and joints.
If you experience prolonged bleeding while taking Plavix
If you cut or injure yourself it may take longer than usual for the bleeding to stop. This is due to the way the medicine works as it prevents blood clots from forming. For minor cuts and injuries, such as cutting yourself or shaving, this usually isn't a problem. However, if you are concerned by your bleeding, you should contact your doctor straightaway (see section 2 'Warnings and precautions').
Other side effects include:
Common side effects (may affect up to 1 in 10 patients):
Diarrhea, abdominal pain indigestion or heartburn.
Uncommon side effects (may affect up to 1 in 100 patients):
Headache, stomach ulcer, vomiting, nausea, constipation, excess gas in the stomach or intestines, rash, itching, dizziness, tingling sensations and numbness.
Rare side effects (may affect up to 1 in 1000 patients):
Vertigo.
Very rare side effects (may affect up to 1 in 10,000 patients):
Jaundice severe abdominal pain with or without back pain; fever, difficulty in breathing sometimes associated with cough; generalized allergic reactions (for example, widespread sensation of heat with sudden general malaise up to fainting); swelling in the mouth; blisters of the skin; skin allergy; pain in the mouth (stomatitis); decrease in blood pressure; confusion; hallucinations; joint pain; muscular pain; changes in taste.
Additionally, your doctor may have identified changes in your blood and urine tests.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist This includes any possible side effects not listed in this leaflet.
You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects you can help provide more information on the safety of this medicine.
Expiry and Retention
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and blister after EXP. The expiry date refers to the last day of that month.
Refer to the storage conditions indicated on the outer packaging. If Plavix is supplied in PVC / PVDC / aluminum blisters, store below 30 ° C. If Plavix is supplied in aluminum / aluminum blisters, the medicinal product does not require any special storage conditions.
Do not use this medicine if you notice any visible signs of deterioration.
Do not throw any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. This will help protect the environment.
Composition and pharmaceutical form
What Plavix contains
The active ingredient is clopidogrel. Each tablet contains 75 mg of clopidogrel (as hydrogen sulfate).
The other ingredients are (see section 2 "Plavix contains lactose" and "Plavix contains hydrogenated castor oil"):
- Tablet core: mannitol (E421), hydrogenated castor oil, microcrystalline cellulose, macrogol 6000 and low-substituted hydroxypropylcellulose,
- Tablet coating: lactose monohydrate (milk sugar), hypromellose (E464), triacetin (E1518), red iron oxide (E 172), titanium dioxide (E 171)
- Polishing agent: carnauba wax.
What Plavix looks like and contents of the pack
Plavix 75 mg film-coated tablets are round, biconvex, pink in color, debossed with the number "75" on one side and the number "1171" on the other side. Plavix is supplied in cartons containing:
- 7, 14, 28, 30, 84, 90 and 100 tablets in PVC / PVDC / aluminum blister or aluminum / aluminum blister,
- 50x1 tablets in PVC / PVDC / aluminum blisters or single-dose aluminum perforated blisters. Not all pack sizes may be marketed.
Source Package Leaflet: AIFA (Italian Medicines Agency). Content published in January 2016. The information present may not be up-to-date.
To have access to the most up-to-date version, it is advisable to access the AIFA (Italian Medicines Agency) website. Disclaimer and useful information.
01.0 NAME OF THE MEDICINAL PRODUCT
PLAVIX 75 MG TABLETS COATED WITH FILM
02.0 QUALITATIVE AND QUANTITATIVE COMPOSITION
Each film-coated tablet contains 75 mg of clopidogrel (as hydrogen sulfate).
Excipients with known effects:
each film-coated tablet contains 3 mg of lactose and 3.3 mg of hydrogenated castor oil.
For the full list of excipients, see section 6.1.
03.0 PHARMACEUTICAL FORM
Film-coated tablet
Pink, round, biconvex engraved with "75" on one side and "1171" on the other side.
04.0 CLINICAL INFORMATION
04.1 Therapeutic indications
Prevention of atherothrombotic events
Clopidogrel is indicated in:
• Adult patients with myocardial infarction (from a few days to less than 35 days), ischemic stroke (from 7 days to less than 6 months) or proven peripheral arterial disease
• Adult patients with acute coronary syndrome:
- acute coronary syndrome without ST segment elevation (unstable angina or myocardial infarction without Q waves), including patients undergoing stent placement following percutaneous coronary intervention, in combination with acetylsalicylic acid (ASA).
- acute coronary syndrome with ST segment elevation in association with ASA in drug therapy patients candidates for thrombolytic therapy.
Prevention of atherothrombotic and thromboembolic events in atrial fibrillation
Clopidogrel in combination with ASA is indicated in the prevention of atherothrombotic and thromboembolic events, including stroke in adult patients with atrial fibrillation who have at least one risk factor for vascular events, unsuitable for treatment with vitamin K antagonists. (AVK) and who have a low risk of bleeding.
For further information see section 5.1.
04.2 Posology and method of administration
Dosage
• Adults and the elderly population
Clopidogrel is given as a single daily dose of 75 mg.
In patients with acute coronary syndrome:
- acute coronary syndrome without ST segment elevation (unstable angina or myocardial infarction without Q waves): Clopidogrel treatment should be started with a single 300 mg loading dose and then continued at 75 mg once daily (in combination with acetylsalicylic acid (ASA) 75 mg -325 mg per day). Since higher doses of ASA have been correlated with a higher bleeding risk, it is recommended that the dose of ASA not exceed 100 mg. The optimal duration of treatment has not been formally established. Clinical trial data support use up to 12 months and maximum benefit was seen at 3 months (see section 5.1).
- ST segment elevation acute myocardial infarction: clopidogrel should be administered as a single daily dose of 75 mg starting with a 300 mg loading dose in combination with ASA, with or without thrombolytics. In patients over 75 years of age, clopidogrel should be initiated without a loading dose. Combination therapy should be started as early as possible after the onset of symptoms and continued for at least 4 weeks. The benefit of combining clopidogrel with ASA beyond four weeks has not been studied in this setting (see section 5.1).
In patients with atrial fibrillation, clopidogrel can be administered as a single daily dose of 75 mg. Treatment with ASA (75-100 mg daily) should be initiated and continued in combination with clopidogrel (see section 5.1).
If a dose is missed:
- within 12 hours of scheduled intake: the patient should take the dose immediately and take the next dose at the usual time.
- if more than 12 hours have passed: the patient should take the next dose at the usual time and should not take a double dose.
• Pediatric population
Clopidogrel should not be used in children due to efficacy concerns. (see section 5.1)
• Kidney failure
Therapeutic experience in patients with renal insufficiency is limited (see section 4.4).
• Hepatic insufficiency
Therapeutic experience in patients with moderate hepatic dysfunction who may have haemorrhagic diathesis is limited (see section 4.4).
Method of administration
Oral use.
The tablet can be taken with or without meals.
04.3 Contraindications
• Hypersensitivity to the active substance or to any of the excipients listed in section 2 or section 6.1.
• Severe hepatic insufficiency.
• Pathological bleeding in progress such as eg. in the presence of a peptic ulcer, or intracranial hemorrhage.
04.4 Special warnings and appropriate precautions for use
Bleeding and haematological pathologies
Because of the risk of bleeding and haematological adverse reactions, the performance of a complete blood count and / or other appropriate tests should be immediately considered whenever clinical symptoms suggestive of bleeding occur during treatment (see section 4.8) As with other antiplatelet drugs, clopidogrel should be used with caution in patients who may be at risk of increased bleeding following trauma, surgery or other pathological conditions and in patients treated with ASA, heparin, glycoprotein inhibitors. IIb / IIIa or non-steroidal anti-inflammatory drugs (NSAIDs) including COX-2 inhibitors, or selective serotonin reuptake inhibitors (SSRIs). Patients should be followed closely for any signs of bleeding, including occult bleeding, in particularly during the first few weeks of treatment and / or after cardiac procedures invasive or surgical interventions. Co-administration of clopidogrel and oral anticoagulants is not recommended as it may result in increased bleeding intensity (see section 4.5).
If a patient is to undergo elective surgery for which antiplatelet activity is temporarily not advisable, clopidogrel use should be discontinued 7 days prior to surgery. Before undergoing any surgery and before taking a new one. medication Patients should advise their doctor and dentist that they are being treated with clopidogrel.Clopidogrel prolongs bleeding time and should be used with caution in patients with bleeding-prone lesions (particularly gastrointestinal and intraocular).
Patients should be advised that the use of clopidogrel (alone or in combination with ASA) could prolong any bleeding and that they should inform their physician of any abnormal bleeding (localization or duration) that may occur.
Thrombotic thrombocytopenic purpura (PTT)
Thrombotic thrombocytopenic purpura (TTP) has been reported very rarely following the use of clopidogrel, sometimes after short exposure. This is characterized by thrombocytopenia and microangiopathic haemolytic anemia associated with or with neurological problems, renal dysfunction or fever.
TTP is a potentially fatal condition that requires immediate treatment including plasmapheresis.
Acquired hemophilia
Acquired haemophilia has been reported following the use of clopidogrel. In the event of isolated activated Partial Thromboplastin Time (aPTT) prolongation with or without ongoing bleeding, acquired haemophilia should be considered. Patients with a confirmed diagnosis of acquired haemophilia should be managed and treated by medical specialists. Treatment with clopidogrel should be discontinued.
Recent ischemic stroke
Due to the lack of data, clopidogrel cannot be recommended during the first 7 days following acute ischemic stroke.
Cytochrome P450 2C19 (CYP2C19)
Pharmacogenetics: When clopidogrel is administered at the recommended dosage in patients poor metabolisers of CYP2C19, the formation of the active metabolite of clopidogrel is reduced and the effect on platelet function is minor. Tests are available to identify a patient's CYP2C19 genotype.
Since clopidogrel is converted to its active metabolite in part by CYP2C19, the use of medicinal products that inhibit the activity of this enzyme is expected to lead to a reduction in the pharmacological levels of the active metabolite of clopidogrel. The clinical relevance of this interaction is uncertain. As a precaution, concomitant use of strong or moderate CYP2C19 inhibitors should be discouraged (see section 4.5 for a list of CYP2C19 inhibitors; see also section 5.2).
Cross-reactions between thienopyridines
Patients should be evaluated for a clinical history of hypersensitivity to thienopyridines (such as clopidogrel, ticlopidine, prasugrel) as cross-reactivity has been reported among thienopyridines (see section 4.8 "Undesirable effects"). Thienopyridines can cause moderate to severe allergic reactions such as rash, angioedema or haematological cross reactions such as thrombocytopenia and neutropenia. Patients who have experienced a previous allergic and / or haematological reaction to one thienopyridine may have an increased risk of developing the same or an "other reaction to" another thienopyridine. Monitoring for signs of hypersensitivity in patients with a known allergy to thienopyridines is advised.
Kidney failure
Therapeutic experience with clopidogrel is limited in patients with renal insufficiency. Clopidogrel should therefore be used with caution in these patients (see section 4.2).
Hepatic insufficiency
Therapeutic experience with clopidogrel is limited in patients with moderate hepatic dysfunction who may have bleeding diathesis. Clopidogrel should therefore be used with caution in these patients (see section 4.2).
Excipients
Plavix contains lactose. Patients with rare hereditary problems of galactose intolerance, the Lapp-lactase deficiency or glucose-galactose malabsorption should not take the medicine.
This medicine contains hydrogenated castor oil which can cause stomach upset and diarrhea.
04.5 Interactions with other medicinal products and other forms of interaction
Oral anticoagulants: co-administration of clopidogrel and oral anticoagulants is not recommended as it may result in increased bleeding intensity (see section 4.4). Although administration of clopidogrel 75 mg / day did not change the pharmacokinetics of S-warfarin or the International Normalized Ratio (INR) in patients on long-term treatment with warfarin, co-administration of clopidogrel and warfarin increases the risk of bleeding. due to the independent effects on haemostasis.
Inhibitors of glycoprotein IIb / IIIa: clopidogrel should be used with caution in patients receiving concomitant glycoprotein IIb / IIIa inhibitors (see section 4.4).
Acetylsalicylic acid (ASA) : ASA does not modify the clopidogrel-mediated inhibition of ADP-induced platelet aggregation; however, clopidogrel potentiates the effect of ASA on collagen-induced platelet aggregation. However, co-administration of ASA 500 mg twice daily for one day did not significantly further prolong clopidogrel-induced bleeding time. A pharmacodynamic interaction is possible between clopidogrel and acetylsalicylic acid, with an increased risk of bleeding. Therefore, concomitant use should be made with caution (see section 4.4). However, clopidogrel and ASA were administered together for up to 1 year (see section 5.1).
Heparin: In a clinical study conducted on healthy subjects, following the administration of clopidogrel no modification of the heparin dose was necessary nor was the effect of heparin on coagulation altered. Co-administration of heparin had no effect on the inhibition of platelet aggregation induced by clopidogrel. A pharmacodynamic interaction is possible between clopidogrel and heparin, with an increased risk of bleeding. Therefore, concomitant use should be made with caution (see section 4.4).
Thrombolytics: the safety of concomitant administration of clopidogrel, fibrin or non-fibrin specific thrombolytic drugs and heparins was studied in patients with acute myocardial infarction.
The incidence of clinically significant bleeding was similar to that observed when thrombolytic drugs and heparin were administered together with ASA (see section 4.8).
NSAIDs: in a clinical study conducted in healthy volunteers, the concomitant administration of clopidogrel and naproxen resulted in increased occult gastrointestinal bleeding.
However, due to the lack of interaction studies with other NSAIDs, it is currently unclear whether there is an increased risk of gastrointestinal bleeding with all NSAIDs. Consequently, co-administration of NSAIDs including COX-2 inhibitors and clopidogrel should be performed with caution (see section 4.4).
Selective Serotonin Reuptake Inhibitors (SSRIs): Since SSRIs affect platelet activation and increase the risk of bleeding, co-administration of SSRIs with clopidogrel should be done with caution.
Other concomitant therapies:
Since clopidogrel is converted to its active metabolite in part by CYP2C19, the use of medicinal products that inhibit the activity of this enzyme is expected to lead to a reduction in the pharmacological levels of the active metabolite of clopidogrel. The clinical relevance of this interaction is uncertain. As a precaution, concomitant use of strong or moderate CYP2C19 inhibitors should be discouraged (see sections 4.4 and 5.2).
Medicinal products that inhibit CYP2C19 include omeprazole and esomeprazole, fluvoxamine, fluoxetine, moclobemide, voriconazole, fluconazole, ticlopidine, ciprofloxacin, cimetidine, carbamazepine, oxicarbazepine and chloramphenicol.
Proton Pump Inhibitors (PPI)
Administration of omeprazole, single dose of 80 mg / day, and of clopidogrel both concomitantly and 12 hours apart, decreased exposure to the active metabolite by 45% (loading dose) and by 40% (maintenance dose). The decrease was associated with a reduction in inhibition of platelet aggregation by 39% (loading dose) and 21% (maintenance dose). similar interaction.
Contradictory data on the clinical implications of this pharmacokinetic (PK) / pharmacodynamic (PD) interaction in terms of major cardiovascular events have been reported in both clinical and observational studies. As a precaution, concomitant use of omeprazole and esomeprazole should be discouraged (see section 4.4).
Less marked reductions in metabolite exposure were observed with pantoprazole and lansoprazole.
Plasma concentrations of the active metabolite were reduced by 20% (loading dose) and 14% (maintenance dose) during concomitant treatment with pantoprazole 80 mg once daily. This was associated with a reduction in mean platelet aggregation inhibition of 15% and 11%, respectively. These results indicate that clopidogrel can be administered with pantoprazole.
There is no evidence that other medicinal products that reduce gastric acidity such as H2 blockers (except cimetidine which is a CYP2C19 inhibitor) or antacids interfere with the antiplatelet activity of clopidogrel.
Other Medicines:
Several other clinical studies have been conducted with clopidogrel and other concomitant therapies to investigate potential pharmacodynamic and pharmacokinetic interactions.
No relevant pharmacodynamic interactions were observed when clopidogrel was administered with atenolol or nifedipine alone or in combination. Furthermore, the pharmacodynamic activity of clopidogrel was not significantly affected by the concomitant administration of phenobarbital or estrogen.
The pharmacokinetics of digoxin and theophylline were not affected by co-administration with clopidogrel. Antacids did not alter the absorption of clopidogrel.
Data from the CAPRIE study indicate that phenytoin and tolbutamide which are metabolised by CYP2C9 can be safely administered concurrently with clopidogrel.
In addition to the information described above on specific drug interactions, interaction studies with clopidogrel and some drugs commonly administered to patients with atherothrombotic disease have not been conducted. However, patients included in clinical trials with clopidogrel received several concomitant therapies including diuretics, beta blockers, ACE inhibitors, calcium channel blockers, cholesterol lowering agents, coronary vasodilators, antidiabetic drugs (including insulin), antiepileptic drugs and glycoprotein IIb / IIIa antagonists with no evidence of clinically significant negative interactions.
04.6 Pregnancy and lactation
Pregnancy
As no clinical data on exposure to clopidogrel in pregnancy are available, it is preferable not to use clopidogrel during pregnancy as a precautionary measure.
Animal studies do not indicate direct or indirect harmful effects with respect to pregnancy, embryonal / fetal development, parturition or postnatal development (see section 5.3).
Feeding time
It is unknown whether clopidogrel is excreted in human milk. Animal studies have shown that clopidogrel is excreted in milk. As a precautionary measure, breastfeeding should not be continued during treatment with Plavix.
Fertility
In animal studies, clopidogrel did not show impaired fertility.
04.7 Effects on ability to drive and use machines
Clopidogrel has no or negligible influence on the ability to drive or use machines.
04.8 Undesirable effects
Summary of the safety profile
Clopidogrel has been evaluated for safety in more than 44,000 patients who have participated in clinical trials, including over 12,000 treated for 1 year or more. In the CAPRIE study, clopidogrel, at a dose of 75 mg / day, was, overall, comparable to ASA 325 mg / day regardless of age, gender and race of the patients. Clinically relevant adverse reactions observed in the CAPRIE, CURE, CLARITY studies , COMMIT and ACTIVE-A are discussed below.
In addition to clinical trial experience, adverse reactions have been spontaneously reported.
Bleeding is the most commonly reported reaction in both clinical trials and post-marketing experience, where it was mainly reported during the first month of treatment.
In the CAPRIE study in both clopidogrel and ASA-treated patients, the overall incidence of any bleeding pattern was 9.3%. The incidence of severe cases was similar for clopidogrel and ASA.
In the CURE study, there was no excess major bleeding with clopidogrel plus ASA in the 7 days following coronary artery bypass grafting in patients who stopped therapy for more than 5 days prior to surgery. in the 5 days prior to bypass surgery, the incidence was 9.6% for clopidogrel plus ASA and 6.3% for placebo plus ASA.
In the CLARITY study, there was an overall increase in bleeding in the clopidogrel plus ASA group compared to the placebo plus ASA group. The incidence of major bleeding was similar across groups. This result was consistent across patient subgroups defined by baseline characteristics and by type of fibrinolytic or heparin therapy.
In the COMMIT study, the overall rate of non-cerebral major bleeding or cerebral bleeding was low and similar in the two groups.
In the ACTIVE-A study, the overall rate of major bleeding was higher in the clopidogrel + ASA group than in the placebo + ASA group (6.7% vs 4.3%). Major bleeds were mainly of extracranial origin in both groups (5.3% in the clopidogrel + ASA group; 3.5% in the placebo + ASA group), mostly occurring in the gastrointestinal tract (3.5% vs 1.8%). Excess intracranial bleeding was observed in the clopidogrel + ASA group compared to the placebo + ASA group (1.4% vs 0.8%, respectively). There were no statistically significant differences between the groups in the rate of fatal bleeding (1.1% in the clopidogrel + ASA group and 0.7% in the placebo + ASA group) and haemorrhagic stroke (0.8% and 0.6% respectively).
Table of adverse reactions
Adverse reactions observed in clinical studies or which were spontaneously reported are shown in the table below. Their frequency is defined using the following conventions: common (≥1 / 100,
* Information regarding clopidogrel with frequency "not known".
Reporting of suspected adverse reactions
Reporting of suspected adverse reactions that occur after authorization of the medicinal product is important. It allows continuous monitoring of the benefit / risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system. "Annex V.
04.9 Overdose
Overdose of clopidogrel can lead to prolonged bleeding time and consequent bleeding complications. If bleeding is observed, appropriate therapy should be considered.
There is no known antidote to the pharmacological activity of clopidogrel. When rapid correction of prolonged bleeding time is required, a transfusion of platelets may reverse the effects of clopidogrel.
05.0 PHARMACOLOGICAL PROPERTIES
05.1 Pharmacodynamic properties
Pharmacotherapeutic group: antiplatelet agents, excluding heparin.
ATC code: B01AC / 04.
Mechanism of action
Clopidogrel is a prodrug, one of its metabolites is an inhibitor of platelet aggregation.
Clopidogrel must be metabolised by CYP450 enzymes to produce the active metabolite that inhibits platelet aggregation.
The active metabolite of clopidogrel selectively inhibits the binding of adenosine diphosphate (ADP) to its platelet P2Y12 receptor, and consequently inhibits ADP-mediated activation of the glycoprotein GPIIb-IIIa complex, and therefore platelet aggregation is inhibited.
Due to the irreversible binding, platelets exposed to clopidogrel are affected for the rest of their life (approximately 7-10 days) and the recovery of normal platelet function occurs with a course dependent on platelet turnover. Platelet aggregation induced by agonists other than ADP is also inhibited by blocking the amplification of platelet activation due to released ADP.
Since the active metabolite is produced by the activity of CYP450 enzymes, some of which are polymorphic or subject to inhibition by other medicinal products, not all patients will have adequate platelet inhibition.
Pharmacodynamic properties
Repeated doses of 75 mg per day produced marked inhibition of ADP-induced platelet aggregation from day one; inhibition progressively increased until stabilizing between day three and seventh. In this steady-state condition the mean inhibition level observed with a dose of 75 mg per day ranged from 40-60%. Platelet aggregation and bleeding time gradually returned to baseline usually within 5 days of stopping treatment.
Clinical efficacy and safety
The safety and efficacy of clopidogrel were evaluated in 5 double-blind studies involving more than 88,000 patients: the CAPRIE study, comparing clopidogrel and ASA, and the CURE, CLARITY, COMMIT and ACTIVE-A comparator studies. between clopidogrel and placebo, both given in combination with ASA and other standard therapies.
Recent myocardial infarction (MI), recent stroke or documented peripheral arterial disease
The CAPRIE study involved 19,185 patients with atherothrombosis manifested by recent myocardial infarction (
Clopidogrel significantly reduced the incidence of new ischemic events (combined end point of myocardial infarction, ischemic stroke and vascular death) compared to ASA. In the intention to treat analysis, 939 events were observed in the clopidogrel group and 1,020 events with ASA, (relative risk reduction (RRR) 8.7%, [95% CI: 0.2 to 16.4]; p = 0.045), which corresponds, for every 1,000 patients treated for 2 years, to 10 additional patients [CI: 0 to 20] who were prevented from new ischemic events. The analysis of total mortality as a secondary endpoint showed no significant difference between clopidogrel (5.8%) and ASA (6.0%).
In the "subgroup analysis performed for qualifying pathology (myocardial infarction, ischemic stroke and peripheral arterial disease) the benefit appeared to be more consistent (reaching statistical significance at p = 0.003) in patients enrolled for peripheral arterial disease (especially for those with history of myocardial infarction) (RRR = 23.7%; CI: 8.9 to 36.2) and less consistent (not significantly different from ASA) in stroke patients (RRR = 7.3%; CI: from - 5.7 to 18.7 [p = 0.258]). In patients enrolled in the study on the sole basis of a recent myocardial infarction, clopidogrel was numerically lower, but not statistically different from ASA (RRR = - 4.0%; CI : - 22.5 to 11.7 [p = 0.639]) In addition, a subgroup analysis for age indicated that the benefit of clopidogrel in patients over 75 years was less than that seen in patients ≤75 years of age.
Since the CAPRIE study was not designed to evaluate efficacy in individual subgroups, it is unclear whether the differences in relative risk reduction for various qualifying conditions are real or due to chance.
Acute coronary syndrome
The CURE study was conducted in 12,562 patients with acute coronary syndrome without ST segment elevation (unstable angina or myocardial infarction without Q waves), who had the onset of their most recent episode of chest pain or symptoms consistent with ischaemia in the 24 hours. Previous hours. Patients were required to have either ECG changes consistent with new ischemia or elevation of cardiac enzymes or troponin I or T at least 2 times the ULN. Patients were randomized to clopidogrel treatment (300 mg loading dose followed 75 mg / day, N = 6259) or placebo (N = 6303), both administered in combination with ASA (75-325 mg once daily) and other standard therapies Patients were treated for up to one year. In the CURE study, 823 patients (6.6%) received concomitant therapy of GPIIb / IIIa receptor antagonists. Heparin was administered in more than 90% of patients and the relative percentage of Pounding between clopidogrel and placebo was not significantly affected by concomitant heparin therapy.
The number of patients experiencing the primary endpoint (cardiovascular death, myocardial infarction, or stroke) was 582 (9.3%) in the clopidogrel group and 719 (11.4%) in the placebo group. , with a 20% relative risk reduction (95% CI 10% to 28%; p = 0.00009) for the clopidogrel group (17% relative risk reduction when patients were treated conservatively, 29 % when undergoing percutaneous transluminal coronary angioplasty (PTCA) with or without stent and 10% when undergoing coronary artery bypass grafting (CABG) New cardiovascular events (primary endpoint) were prevented with a relative risk reduction of 22 % (CI: 8.6 to 33.4), 32% (CI: 12.8 to 46.4), 4% (CI: -26.9 to 26.7), 6% (CI: -33.5 to 34.3) and 14% (CI: -31.6 to 44.2), during study intervals 0-1, 1-3, 3-6, 6-9, and 9- 12 months, respectively Therefore, in addition to 3 months of treatment, the os served in the clopidogrel + ASA group was not further increased while the risk of haemorrhage persisted (see section 4.4).
The use of clopidogrel in CURE was associated with a decrease in the need for thrombolytic treatment (RRR = 43.3%; CI: 24.3% to 57.5%) and GPIIb / IIIa inhibitors (RRR = 18, 2%; CI: 6.5%, 28.3%).
The number of patients experiencing the co-primary endpoint (cardiovascular death, myocardial infarction, stroke or refractory ischaemia) was 1,035 (16.5%) in the clopidogrel group and 1,187 (18.8%) in the placebo group, with a relative risk reduction of 14% (95% CI 6% to 21%, p = 0.0005) for the clopidogrel group. This benefit was mainly determined by a statistically significant reduction in "incidence of myocardial infarction" [287 (4.6%) in the clopidogrel group and 363 (5.8%) in the placebo group]. There was no effect on the rate of re-hospitalization for unstable angina.
The results obtained in populations with different characteristics (e.g. unstable angina or myocardial infarction without Q waves, low or high risk levels, diabetes, need for revascularization, age, sex, etc.) were found to be consistent with the results of the " Primary analysis. In particular, in a post-hoc analysis in 2,172 patients (17% of the total population of the CURE study) who had undergone stent placement (Stent-CURE), the data showed a significant RRR of 26.2 % in favor of clopidogrel over placebo for the co-primary endpoint (cardiovascular death, myocardial infarction, stroke) and a significant RRR of 23.9% for the second co-primary endpoint (cardiovascular death, myocardial infarction, stroke or ischemia Furthermore, the safety profile of clopidogrel in this subgroup of patients did not reveal any particular problems. Therefore, the results obtained by this subgroup are in line with the overall results. ssivi of the study.
The benefit observed with clopidogrel was independent of the use of other acute and long-term cardiovascular therapies (such as heparin / LMWH, glycoprotein IIb / IIIa antagonists, lipid-lowering drugs, beta blockers, and ACE inhibitors). The efficacy of clopidogrel was independent of the dose of ASA (75-325 mg once daily).
In patients with acute ST-segment elevation MI, the safety and efficacy of clopidogrel were evaluated in 2 randomized, double-blind, placebo-controlled studies, CLARITY and COMMIT.
The CLARITY study enrolled 3,491 patients who presented within 12 hours of onset of an ST-segment elevation MI and were candidates for thrombolytic therapy. Patients received clopidogrel (300 mg loading dose, followed by 75 mg / day). , n = 1752) or placebo (n = 1739), both in combination with ASA (150 to 325 mg loading dose, followed by 75-162 mg / day), a fibrinolytic drug and, where necessary, heparin. were observed for 30 days. The primary endpoint was the occurrence of one of the following events: infarct-related artery occlusion, found at pre-discharge angiography, or death, or recurrence of MI before coronary angiography . For patients who did not undergo coronary angiography, the primary endpoint was death or recurrence of MI by day 8 or by hospital discharge. The patient population included 19.7% women and the 29.2% of patients entities aged ≥ 65 years. Overall 99.7% of patients received fibrinolytics (specific fibrin: 68.7%, non-specific fibrin: 31.1%), 89.5% heparin, 78.7% beta blockers, 54.7 % ACE inhibitors and 63% statins.
The incidence of the primary endpoint was fifteen percent (15.0%) in patients in the clopidogrel group and 21.7% in patients in the placebo group, with an absolute reduction of 6.7% and a reduction in 36% risk in favor of clopidogrel (95% CI: 24, 47%; heart attack related parteries. This benefit was consistent across all prespecified subgroups including age and sex, location of infarction and type of fibrinolytic subgroups. or heparin used.
The COMMIT study with 2x2 factorial design enrolled 45,852 patients who presented within 24 hours of onset of suspected MI symptoms, with support for ECG abnormalities (eg, ST segment elevation, ST segment lowering, or blockage). left branch). Patients received clopidogrel (75 mg / day, n = 22.961) or placebo (n = 22.891), in combination with ASA (162 mg / day), for 28 days or until hospital discharge. Co-primary endpoints had death from any cause and the first occurrence of re-heart attack, stroke or death. The population included 27.8% women, 58.4% patients aged ≥ 60 years (26% ≥ 70 years) and 54.5% of patients received fibrinolytics.
Clopidogrel significantly reduced the relative risk of death from any cause by 7% (p = 0.029), and the relative risk of the combination of re-heart attack, stroke or death by 9% (p = 0.002), with an absolute reduction 0.5% and 0.9%, respectively. This benefit was consistent with age, sex and use or otherwise of fibrinolytics and was seen as early as the first 24 hours.
Atrial fibrillation
The ACTIVE-W and ACTIVE-A studies, separate studies within the ACTIVE program, included patients with atrial fibrillation (AF) who possessed at least one risk factor for vascular events. Based on the enrollment criteria, physicians included patients in the ACTIVE-W study if they were eligible for treatment with vitamin K antagonists (AVKs) (such as warfarin). The ACTIVE-A study included patients who could not receive AVK treatment because they were unable or unwilling to undergo the treatment.
The ACTIVE-W study demonstrated that anticoagulant treatment with vitamin K antagonists was more effective than treatment with clopidogrel and ASA.
ACTIVE-A (n = 7,554) is a multicenter, randomized, double-blind, placebo-controlled study comparing clopidogrel 75mg / day + ASA (N = 3,772) with placebo + ASA (N = 3,782). The recommended dose of ASA ranged from 75 to 100 mg / day. Patients were treated for up to 5 years.
Patients randomized to the ACTIVE program were required to have documented AF, e.g. Permanent AF or at least 2 episodes of intermittent AF that had occurred in the past 6 months and must have had at least one of the following risk factors:
• age ≥ 75 years or
• age between 55 and 74 years e
- diabetes mellitus requiring drug therapy o
- previous documented MI or documented coronary heart disease;
• being treated for systemic hypertension;
• previous stroke, transient ischemic attack (TIA) or non-CNS systemic embolism;
• left ventricular dysfunction with left ventricular ejection fraction
• documented peripheral obliterative arteriopathy.
The mean CHADS2 score was 2.0 (range 0-6).
The main exclusion criteria for patients consisted of a peptic ulcer documented in the previous 6 months; previous intracerebral hemorrhage; significant thrombocytopenia (platelet count
Seventy-three percent (73%) of patients enrolled in the ACTIVE-A study were ineligible to take an AVK following medical evaluation, inability to comply with INR (International Normalized Ratio) monitoring, predisposition to fall or suffer trauma head, or specific bleeding risk; for 26% of patients the physician's decision was based on the patient's reluctance to take an VKA.
41.8% of the study population were women. The mean age was 71 years, 41.6% of patients were ≥75 years of age. In total, 23% of patients were treated with antiarrhythmics, 52.1% with beta blockers, 54.6% with ACE inhibitors and 25% with statins.
The number of patients reaching the primary endpoint (time to first stroke, MI, non-CNS systemic embolism, or vascular death) was 832 patients (22.1%) in the clopidogrel + ASA and 924 patients (24.4%) in the placebo + ASA group (relative risk reduction of 11.1%; 95% CI 2.4% -19.1%; p = 0.013), mainly due to the large reduction stroke occurred in 296 patients (7.8%) treated with clopidogrel + ASA and 408 patients (10.8%) treated with placebo + ASA (relative risk reduction of 28.4 %; 95% CI, 16.8% -38.3%; p = 0.00001).
Pediatric population
In an incremental dose study of 86 newborns or infants up to 24 months of age at risk of thrombosis (PICOLO), clopidogrel was evaluated at consecutive doses of 0.01, 0.1 and 0.2 mg / kg in neonates. and in infants and 0.15 mg / kg in neonates only. The dose of 0.2 mg / kg achieved a mean percent inhibition of 49.3% (platelet aggregation induced by 5mcM of ADP), comparable to that of adults taking Plavix 75 mg / day. In a randomized study, double-blind, parallel group (CLARINET), 906 pediatric patients (neonates and infants) with attenuated cyanotic congenital heart disease with systemic pulmonary arterial shunt were randomized to receive clopidogrel 0.2 mg / kg (n = 467) or placebo (n = 439) with concomitant background therapy up to the time of the second surgical phase. The mean time between implantation of the palliative shunt and the first administration of study drug was 20 days. Approximately 88% of patients received concurrent ASA (between 1 and 23 mg / kg / day). There was no significant difference between the groups for the composite primary endpoint of death, shunt thrombosis, or related cardiac intervention before 120 days of age following an event considered to be thrombotic in nature (89 [19.1%] for the clopidogrel group and 90 [20.5%] for the placebo group) (see section 4.2). adverse reaction most frequently reported in both the clopidogrel and placebo groups, however, there was no significant difference in the bleeding rate between the groups. In the long-term safety follow-up of this study, 26 patients with shunts still placed at one year of age received clopidogrel up to 18 months of age. No safety concerns were noted during this long follow-up period.
The CLARINET and PICOLO studies were conducted using a constituted solution of clopidogrel. In a relative bioavailability study in adults, the clopidogrel constituted solution exhibited a comparable degree of absorption and a slightly higher rate of absorption of the major circulating (inactive) metabolite than the licensed tablet.
05.2 Pharmacokinetic properties
Absorption
After single and repeated oral doses of 75 mg / day, clopidogrel is rapidly absorbed. Peak plasma levels of the drug as such (approximately 2.2-2.5 ng / mL after a single 75 mg oral dose) occur approximately 45 minutes after administration. Absorption is at least 50% based on urinary excretion of clopidogrel metabolites.
Distribution
In vitror, clopidogrel and its major (inactive) metabolite bind reversibly to human plasma proteins (98% and 94%, respectively). The bond is not saturable in vitro over a wide range of concentrations.
Biotransformation
Clopidogrel is extensively metabolised by the liver. In vitro And in vivo, clopidogrel is metabolised by two major metabolic pathways: one esterase mediated leading to hydrolysis into its inactive carboxylic acid derivative (85% of the circulating metabolites), and one mediated by multiple P450 cytochromes. Clopidogrel is first metabolised to the intermediate metabolite 2- oxo-clopidogrel Subsequent transformation of the 2-oxo-clopidogrel intermediate metabolite leads to the formation of the active metabolite, a thiol derivative of clopidogrel. In vitro this metabolic pathway is mediated by CYP3A4, CYP2C19, CYP1A2, CYP2B6. The active thiol metabolite that was isolated in vitror, it binds rapidly and irreversibly to platelet receptors, with consequent inhibition of platelet aggregation.
Following administration of a single 300 mg loading dose of clopidogrel, the Cmax of the active metabolite was twice as high as after administration of the 75 mg maintenance dose for 4 days. Cmax is observed approximately 30 to 60 minutes after administration.
Elimination
In humans, following an oral dose of 14C-labeled clopidogrel, approximately 50% is excreted in the urine and approximately 46% in the faeces within 120 hours of dosing. After a single 75 mg dose, clopidogrel has a half-life of approximately 6 hours. The elimination half-life of the major circulating (inactive) metabolite is eight hours after both single and repeated dose administration.
Pharmacogenetics
CYP2C19 is involved in the formation of both the active metabolite and the 2-oxo-clopidogrel intermediate metabolite. Pharmacokinetics of the active metabolite of clopidogrel and antiplatelet effects as measured by platelet aggregation methods ex vivo, vary according to the CYP2C19 genotype.The CYP2C19 * 1 allele is responsible for fully functional metabolism while the CYP2C19 * 2 and CYP2C19 * 3 alleles are not functional. The CYP2C19 * 2 and CYP2C19 * 3 alleles make up the majority of impaired alleles in Caucasian poor metabolisers ( 85%) and in Asians (99%). Other alleles associated with absent or reduced metabolism are less frequent and include CYP2C19 * 4, * 5, * 6, * 7 and * 8. A poor metaboliser will possess two non-functioning alleles The published frequencies for CYP2C19 genotypes belonging to poor metabolisers are approximately 2% for Caucasians, 4% for Blacks and 14% for Chinese Tests are available to identify a patient's CYP2C19 genotype.
A cross-over study of 40 healthy subjects, 10 subjects for each of the 4 CYP2C19 metabolising groups (ultra-rapid, extensive, intermediate and slow), evaluated the pharmacokinetic and antiplatelet response using clopidogrel 300 mg followed by 75 mg / day and 600 mg followed by 150mg / day for a duration of 5 days (steady state) for each group. There was no substantial difference in active metabolite exposure and mean inhibition of platelet aggregation (PAH) between ultra-rapid, extensive, and intermediate metabolisers. In poor metabolisers, exposure to the active metabolite decreased by 63%. 71% compared to extensive metabolisers. Antiplatelet response following a 300 mg / 75 mg clopidogrel dosing regimen was decreased in poor metabolisers with mean PAH (5 μM ADP) by 24% (24 hours) and 37% (day 5) compared to " PAH found in extensive metabolisers by 39% (24 hours) and 58% (day 5) and that observed in intermediate metabolisers by 37% (24 hours) and 60% (day 5).
When poor metabolisers received a dose regimen of 600 mg / 150 mg, the exposure to the active metabolite was higher than the exposure seen in the clopidogrel 300 mg / 75 mg group. In addition, PAH was 32% (24 hours) and 61% (day 5), a value higher than that observed in the group of poor metabolisers treated with the 300 mg / 75 mg dose regimen and was similar to that of the other groups of CYP2C19 metabolisers treated with the 300 mg / 75 mg dose regimen The results from clinical studies did not establish an appropriate dosage for this patient population.
Consistent with the above results, a meta-analysis including 6 studies with a total of 335 subjects treated with clopidogrel at steady state, showed a decrease in exposure to the active metabolite of 28% for intermediate metabolisers and 72% for intermediate metabolisers. poor metabolisers while inhibition of platelet aggregation (5 μM ADP) was decreased with differences in PAH of 5.9% and 21.4% respectively compared to extensive metabolisers.
The influence of the CYP2C19 genotype on clinical outcomes in clopidogrel-treated patients has not been evaluated in prospective, randomized, controlled clinical trials. However, a number of retrospective analyzes exist to evaluate this effect in clopidogrel-treated patients for whom there are genotype results: CURE (n = 2721), CHARISMA (n = 2428), CLARITY-TIMI 28 (N = 227), TRITON-TIMI 38 (N = 1477) and ACTIVE-A (n = 601), and a number of published cohort studies.
In the TRITON-TIMI 38 study and in 3 cohort studies (Collet, Sibbing, Giusti) the combined group of patients with both intermediate and slow metabolisers reported a higher incidence of cardiovascular events (death, myocardial infarction and stroke) or stent thrombosis. compared to extensive metabolisers.
In the CHARISMA study and in a cohort study (Simon), an increased incidence of events was observed only in poor metabolisers compared to extensive metabolisers.
In studies CURE, CLARITY, ACTIVE-A and in one of the cohort studies (Trenk) no increase in the incidence of events was observed based on metaboliser status.
None of these analyzes were adequately sized to detect differences in results in poor metabolisers.
Special populations
The pharmacokinetics of the active metabolite of clopidogrel are unknown in these special populations.
Kidney failure
After repeated daily doses of 75 mg / day of clopidogrel in subjects with severe renal dysfunction (creatinine clearance 5 to 15 ml / min) the inhibition of ADP-induced platelet aggregation was lower (25%) than that observed in healthy subjects, however, the bleeding time prolongation was similar to that seen in healthy subjects who received clopidogrel 75 mg / day. In addition, clinical tolerability was good in all patients.
Hepatic insufficiency
After repeated doses of clopidogrel 75 mg / day for 10 days in patients with severe hepatic impairment, the inhibition of ADP-induced platelet aggregation was similar to that observed in healthy subjects.
The mean prolongation of bleeding time was also similar between the two groups.
Race
The prevalence of CYP2C19 alleles leading to reduced and intermediate CYP2C19 metabolic activity varies by race / ethnicity (see Pharmacogenetics). From the literature, limited data are available in Asian populations to evaluate the clinical implication of genotyping of this CYP on clinical events.
05.3 Preclinical safety data
In non-clinical studies in rats and baboons, modification of liver parameters was the most frequently observed effect. This occurred for doses at least 25 times higher than the corresponding clinical dose of 75 mg / day. administered to humans, and was a consequence of an effect on hepatic metabolic enzymes. No effect of clopidogrel on hepatic metabolic enzymes was observed in humans at therapeutic doses.
At very high doses, poor gastric tolerability (gastritis, gastric erosions and / or vomiting) has been reported in the rat and baboon.
No carcinogenic effect was observed following administration of clopidogrel in mice for 78 weeks and in rats for 104 weeks up to a dose of 77 mg / kg / day (representing at least 25 times the exposure occurring at the clinical dose. of 75 mg / day in humans).
Clopidogrel evaluated in a series of genotoxicity studies in vitro and in vivor, it did not show any genotoxic activity.
Clopidogrel did not show any effect on fertility in male and female rats and did not show any teratogenic effects in either the rat or the rabbit. When administered to lactating rats clopidogrel caused a slight delay in the development of the offspring. Specific pharmacokinetic studies conducted with labeled clopidogrel have shown that the main compound and its metabolites are excreted in milk. Consequently, a direct (mild toxicity) or indirect (poor palatability) effect cannot be excluded.
06.0 PHARMACEUTICAL INFORMATION
06.1 Excipients
Nucleus:
mannitol (E421);
macrogol 6000;
microcrystalline cellulose;
hydrogenated castor oil;
low-substituted hydroxypropylcellulose.
Coating:
hypromellose (E464);
lactose monohydrate;
triacetin (E1518);
titanium dioxide (E171);
red iron oxide (E172).
Polishing agent:
carnauba wax.
06.2 Incompatibility
Not relevant.
06.3 Period of validity
3 years.
06.4 Special precautions for storage
In PVC / PVDC / aluminum blisters, store below 30 ° C.
In aluminum / aluminum blisters, this medicinal product does not require any special storage conditions.
06.5 Nature of the immediate packaging and contents of the package
PVC / PVDC / aluminum blisters or aluminum / aluminum blisters in carton box containing 7, 14, 28, 30, 84, 90 and 100 film-coated tablets.
PVC / PVDC / aluminum blisters or aluminum perforated single-dose blisters in carton box containing 50x1 film-coated tablets.
Not all pack sizes may be marketed.
06.6 Instructions for use and handling
Unused medicine and waste derived from this medicine must be disposed of in accordance with local regulations.
07.0 MARKETING AUTHORIZATION HOLDER
Sanofi Clir SNC
54, rue La Boétie
F-75008 Paris
France
08.0 MARKETING AUTHORIZATION NUMBER
EU / 1/98/069 / 001a - Carton of 28 film-coated tablets in PVC / PVDC / Al blister
034128013
EU / 1/98/069 / 001b - Carton of 28 film-coated tablets in aluminum / aluminum blister
EU / 1/98/069 / 002a - Carton of 50x1 film-coated tablets in PVC / PVDC / Al blister
034128025
EU / 1/98/069 / 002b - Carton of 50x1 film-coated tablets in aluminum / aluminum blister
EU / 1/98/069 / 003a - Carton of 84 film-coated tablets in PVC / PVDC / Al blister
034128037
EU / 1/98/069 / 003b - Carton of 84 film-coated tablets in aluminum / aluminum blister
EU / 1/98/069 / 004a - Carton of 100 film-coated tablets in PVC / PVDC / Al blister
EU / 1/98/069 / 004b - Carton of 100 film-coated tablets in aluminum / aluminum blister
EU / 1/98/069 / 005a - Carton of 30 film-coated tablets in PVC / PVDC / Al blister
EU / 1/98/069 / 005b - Carton of 30 film-coated tablets in aluminum / aluminum blister
EU / 1/98/069 / 006a - Carton of 90 film-coated tablets in PVC / PVDC / Al blister
EU / 1/98/069 / 006b - Carton of 90 film-coated tablets in aluminum / aluminum blister
EU / 1/98/069 / 007a - Carton of 14 film-coated tablets in PVC / PVDC / Al blister
EU / 1/98/069 / 007b - Carton of 14 film-coated tablets in aluminum / aluminum blister
EU / 1/98/069 / 011a - Carton of 7 film-coated tablets in PVC / PVDC / Al blister
EU / 1/98/069 / 011b - Carton of 7 film-coated tablets in aluminum / aluminum blister
09.0 DATE OF FIRST AUTHORIZATION OR RENEWAL OF THE AUTHORIZATION
Date of first authorization: 15 July 1998
Date of last renewal: July 15, 2008
10.0 DATE OF REVISION OF THE TEXT
D.CCE October 2015