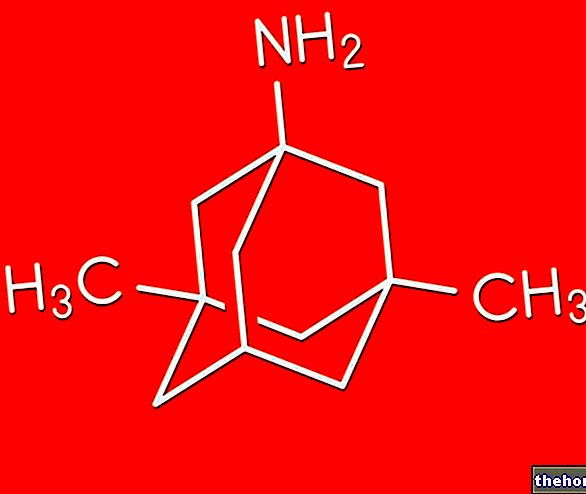
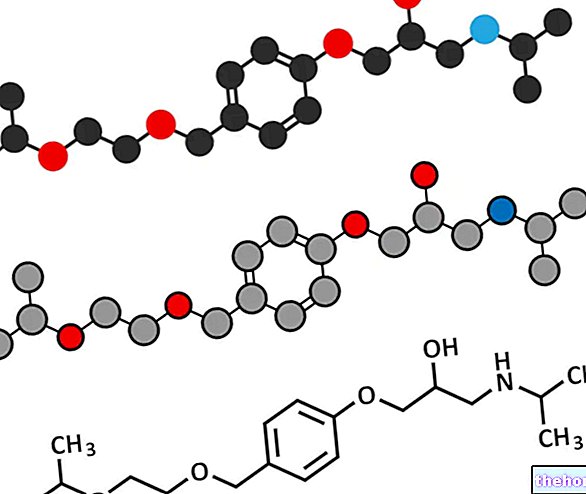
Active ingredients: Fluvoxamina (Fluvoxamine maleate)
FEVARIN 50 mg and 100 mg film-coated tablets
Why is Faverin used? What is it for?
FEVARIN belongs to a class of medicines called selective serotonin reuptake inhibitors (SSRIs). Faverin contains a substance called fluvoxamine. This is an antidepressant and is used to treat depression (major depressive episode).
FEVARIN can also be used to treat people with obsessive compulsive disorder (OCD).
Contraindications When Faverin should not be used
Do not use Faverin if any of the conditions below apply to you:
- if you are allergic (hypersensitive) to fluvoxamine or any of the other ingredients in the tablet (see section "Further information")
- if you are using medicines called monoamine oxidase inhibitors (MAOIs) sometimes prescribed to treat depression or anxiety, including linezolid (an antibiotic which is also a MAOI).
Fluvoxamine treatment should be started at least 2 weeks after stopping an irreversible MAOI. However, fluvoxamine treatment after stopping certain reversible MAOIs can be started the next day. In exceptional cases, linezolid (an antibiotic that is also a MAOI) can be used concomitantly with fluvoxamine as long as your doctor can monitor it closely.
Your doctor will advise you on how to start using Faverin once your MAOI treatment has stopped.
- If you are using tizanidine, a medicine often used as a muscle relaxant
- If you are breast-feeding If any of the above apply to you, do not take Faverin and talk to your doctor.
Precautions for use What you need to know before taking Faverin
Pay particular attention:
Talk to your doctor or pharmacist before taking your medicine if:
- recently had a heart attack
- is pregnant or could be
- has epilepsy
- you have had bleeding problems in the past or if you regularly use medicines that increase the risk of bleeding, such as common pain relievers
- have diabetes
- are being treated with electroconvulsive therapy (ECT)
- have ever had mania (feeling euphoric or overexcited)
- have liver or kidney problems
- have high eye pressure (glaucoma)
- you are under 18 (see also section 3 "How to take Faverarin")
If any of the above apply to you, your doctor will tell you if it is safe for you to start taking Fevarin.
Occasionally, restless thoughts such as inability to sit or stand still (akathisia) may occur or may worsen during the first few weeks of Fevarin treatment, as long as the antidepressant has not worked. Tell your doctor immediately if they occur. such symptoms.A dosage adjustment may therefore be helpful.
Thoughts of suicide and worsening of your depression or anxiety disorders
If you are depressed and / or have anxiety disorders you can sometimes have thoughts of harming or killing yourself. These thoughts may increase at the start of treatment with antidepressants as these medicines take some time to work, usually two weeks. but sometimes more.
You are more likely to think like this:
- if you have previously had thoughts of harming or killing yourself
- if you are a young adult. Information from clinical trials has shown an increased risk of suicidal behavior in adults less than 25 years of age with psychiatric disorders being treated with an antidepressant.
If you have thoughts of harming or killing yourself at any time, contact your doctor or go to a hospital immediately.
It may be helpful to tell a relative or close friend that you are depressed or have an anxiety disorder and ask them to read this leaflet. You can ask them to let you know if they think your depression or anxiety is getting worse. or if they are concerned about changes in their behavior.
Tell your doctor immediately if you have any distressing thoughts or experiences.
Use in children and adolescents under the age of 18
Children and adolescents under 18 years of age should not take this medicine unless they are being treated for obsessive compulsive disorder (OCD). This is because Faverin is not used to treat depression in patients under 18 years of age.
People under 18 who use this type of medicine have an increased risk of side effects, such as suicide attempt, suicidal thoughts and hostility such as aggression, oppositional behavior and anger.
If your doctor has prescribed Faverin for a patient under the age of 18 and you want to discuss this, please contact your doctor again. You should inform your doctor if any of the symptoms described above appear or worsen during the treatment with Faverin of a patient under 18 years of age.
It is also not known whether taking Faverin under the age of 18 will have a long-term effect on growth, maturation and development of intelligence or behavior.
Interactions Which drugs or foods can modify the effect of Faverin
- During treatment with Faverin, you should not start using the herbal preparation St. John's Wort as it may increase the side effects. If you are already taking St John's wort at the start of treatment with Faverin, stop taking it and tell your doctor at your next visit.
- If you are taking or have taken within the past two weeks a medicine to treat depression or anxiety, or if you have schizophrenia, check with your doctor or pharmacist.
Your doctor or pharmacist will check if you are using any other medicines to treat your depression or related disorders; these may include:
- benzodiazepines
- tricyclic antidepressants
- neuroleptics or antipsychotics
- lithium
- tryptophan
- monoamine oxidase inhibitors (MAOIs) such as moclobemide
- selective serotonin reuptake inhibitors (SSRIs) such as citalopram
Your doctor will tell you if it is safe for you to start using Faverin.
You should also tell your doctor or pharmacist if you are using any of the medicines listed below:
- aspirin (acetylsalicylic acid) or medicines like aspirin, used to treat pain and inflammation (arthritis)
- cyclosporine, used to reduce the activity of the immune system
- methadone, used to treat pain and withdrawal symptoms
- mexiletine, used to treat irregular heart rhythms
- phenytoin or carbamazepine, used to treat epilepsy
- propanolol, used to treat high blood pressure and heart disease
- ropinirole, for Parkinson's disease
- a "triptan" used to treat migraines, such as sumatriptan
- terfenadine, used to treat allergies. Faverin should not be used together with terfenadine
- sildenafil, used to treat erectile dysfunction
- theophylline, used to treat asthma and bronchitis
- tramadol, a pain reliever
- warfarin, nicumalone, or any other drug used to prevent blood clots
If you are using or have recently used any of the medicines listed above, and have not yet discussed them with your doctor, please come back to him and ask what to do. Your dose may need to be changed or you may need a different medicine.
Tell your doctor or pharmacist if you are taking or have taken any other medicines - including those obtained without a prescription. These also include herbal medicines.
Taking Faverin with food and drink
- Do not drink alcohol if you are taking this medicine, as alcohol works together with Faverin making you sleepy and not very alert.
- If you normally take a lot of tea, coffee and caffeinated drinks you may have symptoms such as shaking of the hands, malaise, rapid heart rate (palpitations), restlessness and difficulty sleeping (insomnia). By decreasing the caffeine content, these symptoms may disappear.
Warnings It is important to know that:
Pregnancy, breastfeeding and fertility
Ask your doctor or pharmacist for advice before taking any medicine.
Pregnancy
There is only limited experience with the use of fluvoxamine during pregnancy.
Do not take fluvoxamine if you are pregnant unless your doctor considers it absolutely necessary.
If you are already taking fluvoxamine and planning to become pregnant or father a child, ask your doctor for advice to decide if alternative treatment is necessary or appropriate.
. Fluvoxamine has been shown to reduce sperm quality in animal studies. In theory this could have an effect on fertility but so far the impact on fertility has not been observed.
Make sure your midwife and / or doctor know you are on fluvoxamine. Medicines such as fluvoxamine, when taken during pregnancy, particularly in the last 3 months of pregnancy, may increase the risk of a serious condition in babies, called persistent pulmonary hypertension of the newborn (PPHN), which causes the baby to breathe faster and cause a bluish appearance. These symptoms usually appear in the first 24 hours after birth. If this happens to your baby you should immediately inform your midwife or doctor.
You should not abruptly stop fluvoxamine treatment. If you are taking fluvoxamine in the last 3 months of pregnancy, your baby may have other symptoms at birth in addition to breathing problems or blue skin, such as inability to sleep or feed properly, body too hot or too cold, malaise, crying prolonged, stiff or soft muscles, lethargy, tremor, agitation or convulsions. If your baby has any of these symptoms after birth, tell your doctor right away.
Feeding time
Fluvoxamine passes into breast milk. There is a risk of having an effect on the baby, so you should discuss this with your doctor who will decide whether you should stop breastfeeding or fluvoxamine therapy.
Driving and using machines
You can drive and use machines during treatment, as long as this medicine does not make you drowsy.
Dose, Method and Time of Administration How to use Fevarin: Posology
How much Faverin to take
Always take Faverin exactly as your doctor has told you. If in doubt, consult your doctor or pharmacist.
Usual starting dose for adults (18 years and older):
For the treatment of depression:
- Start with 50 or 100 mg per day, taken in the evening
For the treatment of OCD:
- Start with 50 mg per day, preferably in the evening
If after a couple of weeks you don't start to feel better, talk to your doctor who will recommend you. Your doctor may decide to gradually increase the dose.
The maximum recommended daily dose is 300 mg.
If your doctor advises you to take more than 150 mg per day, do not take them all at once, but ask your doctor when to take them.
Usual dose for children and adolescents with OCD - OCD (ages 8 and up):
Start with 25 mg (half a tablet) per day. Your doctor may increase the dose by 25 mg step by step every 4-7 days, based on tolerability, until an effective dose is reached.
The maximum daily dose is 200 mg.
If your doctor advises you to take more than 50 mg per day, do not take them all at once but ask your doctor when to take them. If the dose is not divided equally, the higher dose should be administered at bedtime at night.
Children and adolescents under the age of 18 should not take this medicine to treat depression. This medicine should be prescribed for children and adolescents for obsessive compulsive disorder (OCD) only.
How to take Faverin
Swallow the tablets with water. Don't chew them
You can divide the tablets in half if your doctor has told you to.
How long does it take to act?
Faverin may take some time to start working. Some patients do not feel improvement in the first 2 or 3 weeks of treatment.
Keep taking your tablets until your doctor tells you to stop. Even when you start to feel better, your doctor may want you to continue taking the tablets for some time, at least six months, to be sure the treatment has worked completely.
Do not stop taking Faverin too quickly.
You may have withdrawal symptoms such as:
- agitation and anxiety
- confusion
- diarrhea
- trouble sleeping
- dizziness
- emotional instability
- headache
- irritability
- nausea and / or vomiting
- palpitations (rapid heart rhythm)
- sensitivity disturbances (such as electric shock sensation or visual disturbances)
- sweating
- tremors
When you stop taking FEVARIN, your doctor will help you reduce the dose slowly over a few weeks or months and this should help reduce the occurrence of withdrawal symptoms. For most people, symptoms of discontinuation of Fevarin are mild and resolve on their own within 2 weeks. For some people, these symptoms may be more severe or last longer.
If you have withdrawal symptoms while you are stopping taking the tablets, your doctor may decide that you need to stop taking them more slowly. If you have severe withdrawal symptoms when you stop taking Faverin, see your doctor. He may ask you to start taking the tablets again and stop taking them more slowly (see also section 4 "Possible side effects").
If you have any symptoms upon stopping treatment, please contact your doctor.
Overdose What to do if you have taken too much Faverin
If you take more Faverin than you should
If you or someone else has ingested too much FEVARIN (overdose), contact a doctor or go to a hospital as soon as possible. Take the medicine pack with you.
Symptoms of overdose include, but are not limited to, nausea, vomiting, diarrhea and sleepiness or dizziness.
Heart-related events (slow or fast heart beat, low blood pressure), liver problems, seizures and coma have also been reported.
If you forget to take Faverin
If you forget to take a tablet, wait until the next dose is due. Do not take a double dose to make up for a forgotten dose.
If you have any other questions about the use of this product, ask your doctor or pharmacist.
Side Effects What are the side effects of Fevarin
Like all medicines, FEVARIN can cause side effects (unwanted effects or reactions), although not everybody gets them.
The frequencies of the observed side effects are defined as follows:
Side effects related to this type of medicine
Occasionally suicidal or self-harming thoughts may occur or increase in the first few weeks of Fevarin treatment, until the antidepressant has worked.
Tell your doctor immediately if you have any distressing thoughts or experiences.
If you have several symptoms at the same time you may have one of the rare conditions listed below:
- Serotonin syndrome: if you have sweating, muscle stiffness or spasms, unsteadiness, confusion, irritability or severe agitation
- Neuroleptic Malignant Syndrome: if you have muscle stiffness, high temperature, confusion and other associated symptoms
- SIADH: if you feel tired, weak or confused and have sore, stiff or out of control muscles
Stop taking Faverin and contact your doctor immediately.
If you get unusual bruises or red spots on your skin or if you vomit blood or if you find blood in your stool, contact your doctor for advice.
Withdrawal of fluvoxamine (especially if abrupt) commonly leads to withdrawal symptoms (see section 3 Withdrawal symptoms).
Sometimes patients have mild nausea as soon as Faverin starts to work. Although the feeling of nausea is not pleasant, it should soon pass if you continue to take your tablets as prescribed. It may take a few weeks.
Side effects specifically related to Faverin
Common side effects:
agitation
anxiety
constipation
diarrhea
trouble sleeping
dizziness
dry mouth
fast heart rhythm
drowsiness (lethargy)
malaise
headache
indigestion
loss of appetite
nervousness
stomach pain
sweating
tremor
muscle weakness (asthenia)
He retched
Uncommon side effects:
allergic skin reactions (including swelling of the face, lips or tongue, rash or itching)
confusion
delayed ejaculation
dizziness on standing up too quickly
hallucinations
lack of coordination
pain in the muscles or joints
Rare side effects:
seizures liver problems
mania (feeling euphoric or overexcited)
sensitivity to sunlight
unexpected leaking of milk from the nipple
Other reported side effects:
akathisia (inability to sit still)
change in taste
anorgasmia (failure to reach orgasm)
for female patients: menstruation-related disorders (monthly bleeding)
urinary disorders (such as needing to urinate frequently during the day and / or night, sudden loss of urine control during the day and / or night, or inability to urinate)
paraesthesia (tingling or numbness)
glaucoma (high eye pressure)
dilated pupils
increase in the hormone prolactin (hormone that stimulates milk production in breastfeeding women)
fluctuations in the pes
An increased risk of bone fractures has been observed in patients taking this type of medicine.
Undesirable effects during treatment for OCD in children and adolescents with frequencies not indicated:
mania (feeling euphoric or overexcited)
agitation
convulsions
difficulty sleeping (insomnia)
lack of strength (asthenia)
hyperactivity (hyperkinesis)
drowsiness
indigestion
If any of the side effects gets serious or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Expiry and Retention
- Keep Faverin out of the reach and sight of children.
- Do not use the tablets after the expiry date (EXP) printed on the carton and blister.
- Do not store above 25 ° C.
If your doctor stops taking you, return the unused tablets to a pharmacist.
Do not throw any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. This will help protect the environment.
What Faverin 50 mg and Faverin 100 mg contains
The active ingredient is fluvoxamine maleate.
Each 50 mg tablet contains 50 mg of fluvoxamine maleate.
Each 100 mg tablet contains 100 mg of fluvoxamine maleate. The other ingredients are: mannitol (E421), maize starch, pregelatinised starch, sodium stearyl fumarate, anhydrous colloidal silica, hypromellose, macrogol 6000, talc and titanium dioxide (E171).
What Faverin looks like and contents of the pack
Fevarin 50 mg tablet is white to off-white, round, film-coated tablet debossed on one side of the tablet with "291" on both sides of the score line.
Fevarin 100 mg tablet is white to off-white, oval film-coated tablet debossed with "313" on both sides of the score line.
Faverin 50 mg is available in packs of 5, 10, 20, 30, 50, 60, 90, 100 and 250 tablets.
Fevarin 100 mg is available in packs of 15, 20, 30, 50, 60, 90, 100, 120 and 250 tablets.
Not all pack sizes may be marketed.
Source Package Leaflet: AIFA (Italian Medicines Agency). Content published in January 2016. The information present may not be up-to-date.
To have access to the most up-to-date version, it is advisable to access the AIFA (Italian Medicines Agency) website. Disclaimer and useful information.
01.0 NAME OF THE MEDICINAL PRODUCT
FEVARIN 50 MG TABLETS COATED WITH FILM
02.0 QUALITATIVE AND QUANTITATIVE COMPOSITION
One tablet contains 50 mg of fluvoxamine maleate.
For the full list of excipients, see section 6.1.
03.0 PHARMACEUTICAL FORM
Film-coated tablet.
Round, biconvex, scored, white to off-white film-coated tablets debossed on one side of the tablet with "291" on both sides of the score.
The tablet can be divided into equal halves.
04.0 CLINICAL INFORMATION
04.1 Therapeutic indications
Major depressive episode.
Obsessive Compulsive Disorder (OCD).
04.2 Posology and method of administration
Depression
Adults
The recommended dose is 100 mg per day. Patients should initiate treatment with 50 or 100 mg in a single evening dose. Dosage should be monitored and adjusted, if necessary, within 3-4 weeks of initiation of treatment and thereafter based on clinical judgment. Although the risk of side effects may potentially increase at higher doses, if the response is insufficient after a few weeks of the recommended dose, some patients may benefit from gradually increasing the dose up to a maximum of 300 mg per day. (see section 5.1). Dosages up to 150 mg can be administered as a single dose, preferably in the evening. It is recommended that a total daily dose greater than 150 mg is divided into 2 or 3 administrations.
Dosage adjustments should be made with caution on an individual basis in order to administer the lowest effective dose to patients.
Patients with depression should be treated for a period of at least 6 months to ensure freedom from symptoms.
Children / adolescents
Faverin should not be used in children and adolescents under 18 years of age for the treatment of major depressive episode.
The efficacy and safety of Faverin have not been established in the treatment of pediatric major depressive episode (see section 4.4).
Obsessive Compulsive Disorder
Adults
The recommended dose is between 100 and 300 mg per day. Patients should start treatment with 50 mg per day. Although the risk of undesirable effects may potentially increase at higher doses, if the response is insufficient after a few weeks of dosing the recommended dose, some patients may benefit from gradually increasing the dose up to 300 mg per day (see section 5.1). Dosages up to 150 mg can be administered as a single dose, preferably in the evening. It is recommended that a total daily dose greater than 150 mg is divided into 2 or 3 administrations. If a good therapeutic response is achieved, treatment can be continued at an individually adjusted dosage.
Although there are no systematic studies that can establish the duration of treatment with fluvoxamine, given the chronic nature of OCD, it is reasonable to continue treatment beyond 10 weeks in responding patients. Dosage should be carefully tailored on an individual basis to allow the patient to receive the lowest effective dose. The need for treatment should be reassessed periodically. In patients who respond to drug therapy, some clinicians consider concomitant behavioral therapy to be helpful.
Long-term efficacy (beyond 24 weeks) in OCD has not been demonstrated.
Children / adolescents
In children over 8 years and adolescents, limited data is available at a dose of up to 100 mg twice daily for 10 weeks. The starting dose is 25 mg per day. Increase the dosage by 25 mg every 4-7 days based on tolerability until an effective dose is reached.
The maximum dose in children should not exceed 200 mg / day. (For further details see sections 5.1 and 5.2). It is recommended that a total daily dose greater than 50 mg be divided into two divided doses. If the two divided doses are not the same, the higher dose should be given at bedtime.
Withdrawal symptoms occurring after discontinuation of the fluvoxamine
Abrupt discontinuation of treatment should be avoided. When fluvoxamine treatment needs to be discontinued, the dose should be gradually reduced over at least one to two weeks to reduce the risk of withdrawal symptoms (see sections 4.4 and 4.8).
If intolerable symptoms occur following a decrease in dosage or after discontinuation of treatment, then resuming the previously prescribed dose may be considered. Thereafter, the doctor may continue to decrease the dosage, but more gradually.
Hepatic or renal insufficiency
Patients with hepatic or renal insufficiency should start on a low dose and be carefully monitored.
Method of administration
Fluvoxamine tablets should be swallowed with water and not chewed.
04.3 Contraindications
Fevarin tablets are contraindicated in combination with tizanidine and monoamine oxidase inhibitors (MAOIs) (see sections 4.4 and 4.5).
Fluvoxamine treatment can be initiated:
- two weeks after stopping an irreversible MAOI or
- the day after stopping a reversible MAOI (e.g. moclobemide, linezolid).
See section 4.4 for precautions in exceptional cases where linezolid has to be administered in combination with fluvoxamine.
At least one week should elapse between discontinuation of fluvoxamine and initiation of therapy with any MAOI.
Hypersensitivity to the active substance or to any of the excipients.
04.4 Special warnings and appropriate precautions for use
Suicide / suicidal thoughts or clinical worsening
Depression is associated with an increased risk of suicidal thoughts, self harm and suicide (suicide / related events). This risk persists until significant remission occurs. As improvement may not occur during the first or immediate weeks of treatment, patients should be monitored closely until improvement occurs. It is generally clinical experience that the risk of suicide may increase in the early stages of improvement.
Other psychiatric conditions for which Faverin is prescribed may also be associated with an increased risk of suicidal behavior. Furthermore, these conditions can be associated with major depressive disorder. Therefore, the same precautions followed when treating patients with other psychiatric disorders should be observed when treating patients with major depressive disorders.
Patients with a history of suicide-related events, or who exhibit a significant degree of suicidal ideation prior to initiation of treatment, are at increased risk of suicidal thoughts or suicide attempts, and should be closely monitored during treatment. of clinical trials conducted with antidepressant drugs compared with placebo in adult patients in the therapy of psychiatric disorders, showed an increased risk of suicidal behavior in the age group below 25 years of patients treated with antidepressants compared to placebo.
Drug therapy with antipressants should always be associated with close surveillance of patients, particularly those at high risk, especially in the initial stages of treatment and after dose changes.
Patients (and caregivers) should be advised of the need to monitor and report immediately to their physician any clinical worsening, the onset of suicidal behavior or thoughts, or unusual changes in behavior.
Pediatric population
Fluvoxamine should not be used to treat children and adolescents under 18 years of age with the exception of patients with OCD. Suicidal behaviors (suicide attempts and suicidal thoughts) and hostility (predominantly aggression, oppositional behavior and anger) were observed more frequently in clinical trials in children and adolescents treated with antidepressants than in those treated with placebo. If, based on medical needs, a decision to treat is nevertheless taken, the patient should be carefully monitored for the appearance of suicidal symptoms.
In addition, there is a lack of long-term safety data for children and adolescents regarding growth, maturation and cognitive and behavioral development.
Geriatric population
Data in elderly subjects do not suggest clinically significant differences in normal daily dosages compared to younger subjects. However, dosage escalation should occur more slowly in the elderly and dosage should always be established with caution.
Hepatic and renal impairment
Patients with hepatic or renal impairment should start on a low dose and be carefully monitored.
Fluvoxamine treatment has rarely been associated with an increase in liver enzymes, usually accompanied by clinical symptoms. In such cases the treatment should be stopped.
Withdrawal symptoms occurring after discontinuation of the fluvoxamine
Discontinuation symptoms after discontinuation of treatment are common, particularly if discontinuation is abrupt (see section 4.8). In clinical studies, adverse reactions related to treatment discontinuation were seen in approximately 12% of patients treated with fluvoxamine, similar to the incidence observed in patients treated with placebo. The risk of withdrawal symptoms may depend on several factors including the duration, the dose used for therapy and the rate of dose reduction.
Dizziness, sensory disturbance (including paraesthesia, visual disturbances and electric shock sensations), sleep disturbances (including insomnia and intense dreams), agitation and anxiety, irritability, confusion, emotional instability, nausea and / or vomiting and diarrhea, sweating and palpitations , headache and tremor are the most commonly reported reactions. Generally, these symptoms are mild to moderate in intensity; however in some patients the intensity may be severe. These symptoms mostly occur during the first few days after discontinuation of treatment, but there have been very rare reports of these symptoms in patients who have inadvertently forgotten to take a dose. symptoms are self-limiting and usually resolve within 2 weeks, although in some people they may last longer (2-3 months or more).
It is therefore recommended that the dose of fluvoxamine be progressively reduced over several weeks or months prior to discontinuation of treatment, depending on the patient's needs (see "Withdrawal symptoms occurring after discontinuation of fluvoxamine" section 4.2).
Psychiatric disorders
Fluvoxamine should be used with caution in patients with a history of mania / hypomania. Fluvoxamine should be discontinued in any patient experiencing a manic phase.
Akathisia / psychomotor restlessness
The use of fluvoxamine has been associated with the onset of akathisia, characterized by restlessness, which depending on the subject, can be unpleasant or distressing and the need to move, often accompanied by the inability to sit or stand still. These symptoms are more likely during the first few weeks of treatment. In patients who develop these symptoms, increasing the dosage may be harmful.
Disorders of the nervous system
Although fluvoxamine has not been shown to possess proconvulsive properties in animal studies, caution is recommended when administering the drug to patients with a history of seizure disorders. Administration of fluvoxamine should be avoided in patients with unstable epilepsy and patients with controlled epilepsy should be carefully monitored.
If seizures occur or if the frequency of seizures increases, treatment with fluvoxamine should be discontinued.
Onset of serotonin syndrome or neuroleptic malignant syndrome-like events associated with fluvoxamine treatment has rarely been reported, especially when fluvoxamine is given in combination with other serotonergic and / or neuroleptic drugs. As these syndromes may lead to a potential risk for life, fluvoxamine treatment should be discontinued upon the onset of such events (characterized by a range of symptoms such as hyperthermia, rigidity, myoclonus, autonomic instability with possible rapid fluctuations in vital signs, mental status changes including confusion, irritability, extreme agitation with progression to delirium and coma) and symptomatic supportive treatment should be initiated.
In exceptional circumstances, linezolid (an antibiotic that is also a relatively weak nonselective reversible MAOI) can be given in combination with fluvoxamine provided there are facilities for close observation and management of serotonin syndrome symptoms and blood pressure monitoring (see section 4.3 and 4.5). If such symptoms occur, the physician should consider stopping the treatment of one or both medicines.
Metabolism and nutrition disorders
As with other SSRIs (Selective Serotonin Reuptake Inhibitors), hyponatraemia that appears reversible after discontinuation of fluvoxamine has rarely been reported. Some cases may have been caused by the syndrome of inappropriate antidiuretic hormone secretion.
Most of the reports come from elderly patients.
Glycemic control may be impaired (e.g. hyperglycemia, hypoglycemia, impaired glucose tolerance), particularly in the early stages of treatment. If fluvoxamine is given to patients with a known history of diabetes mellitus, dosage adjustment of antidiabetic drugs may be necessary.
Eye disorders
Mydriasis has been reported in association with SSRIs such as fluvoxamine. Therefore, caution should be exercised when prescribing fluvoxamine to those patients with increased intraocular pressure or those at risk of acute narrow-angle glaucoma.
Hematological disorders
The following bleeding disorders have been reported with SSRIs: gastrointestinal bleeding, gynecological bleeding, and other cutaneous or mucosal bleeding. Caution is advised in patients taking SSRIs, particularly in elderly patients and in patients concurrently using drugs known to affect platelet function (e.g. atypical antipsychotics and phenothiazines, most tricyclic antipressants, acetylsalicylic acid, non-steroidal anti-inflammatory drugs) or drugs that increase the risk of bleeding, as well as in patients with a history of bleeding and in those with predisposing conditions (e.g. thrombocytopenia or coagulation disorders).
Heart ailments
Fluvoxamine should not be administered in combination with terfenadine, astemizole or cisapride as plasma concentrations may be increased resulting in an increased risk of QT prolongation / Torsade de Pointes.
Due to lack of clinical experience, special attention is recommended in the post-acute phase of myocardial infarction.
Electroconvulsive therapy (ECT)
Clinical experience of co-administration of fluvoxamine and ECT is limited and therefore caution is recommended.
04.5 Interactions with other medicinal products and other forms of interaction
Fluvoxamine should not be administered in combination with MAOIs (see also sections 4.3 and 4.4).
Fluvoxamine is a potent inhibitor of CYP1A2 and to a lesser extent CYP2C and CYP3A4. Drugs which are extensively metabolised via these isoenzymes are eliminated more slowly and may reach higher plasma concentrations when co-administered with fluvoxamine. This is particularly relevant for drugs with a narrow therapeutic index. Patients should be monitored closely and, if necessary, dose adjustment of these drugs is recommended.
Fluvoxamine has marginal inhibitory effects on CYP2D6 and does not appear to affect non-oxidative metabolism or renal excretion.
CYP1A2
There was an increase in the previously stable plasma levels of tricyclic antidepressants (such as clomipramine, imipramine and amitriptyline) and neuroleptics (such as clozapine, olanzapine and quetiapine) which are extensively metabolised by cytochrome P450 1A2 when administered in combination with fluvoxamine. If fluvoxamine treatment is initiated, a decrease in the dose of these drugs should be considered.
Patients concurrently taking fluvoxamine and drugs metabolised via CYP1A2 with a narrow therapeutic index (such as tacrine, theophylline, methadone and mexiletine) should be closely monitored and, if necessary, dose adjustment of these drugs is recommended.
There have been isolated reports of cardiac toxicity when fluvoxamine was used in combination with thioridazine.
As the plasma concentrations of propranolol increase when it is used in combination with fluvoxamine, it may be necessary to reduce the dose of propranolol.
Plasma caffeine levels are likely to increase during co-administration with fluvoxamine. Therefore, patients who consume large quantities of caffeinated beverages should reduce their consumption when treated with fluvoxamine and adverse reactions from caffeine (such as tremor, palpitations, nausea, restlessness, insomnia) occur.
Since plasma concentrations of ropinirole may increase in association with fluvoxamine thereby increasing the risk of overdose, it may be necessary to monitor and reduce the dose of ropinirole during treatment with fluvoxamine and after its discontinuation.
CYP2C
Patients concurrently taking fluvoxamine and CYP2C metabolised drugs with a narrow therapeutic index (such as phenytoin) should be closely monitored and, if necessary, dose adjustment of these drugs is recommended.
Warfarin
When co-administered with fluvoxamine, the plasma concentration of warfarin was significantly increased and the prothrombin time was lengthened.
Cytochrome P-450 isoenzymes involved in warfarin metabolism include 2C9, 2C19, 2C8, 2C18, 1A2 and 3A4. 2C9 is probably the major form of human hepatic P-450 that modulates the anticoagulant activity of warfarin in vivo.
CYP3A4
Terfenadine, astemizole, cisapride, sildenafil (see also section 4.4).
Patients concurrently taking fluvoxamine and CYP3A4 metabolised drugs with a narrow therapeutic index (such as carbamazepine and cyclosporine) should be closely monitored and, if necessary, dose adjustment of these drugs is recommended.
Plasma levels of benzodiazepines metabolised by oxidation (such as triazolam, midazolam, alprazolam and diazepam) are likely to increase when these drugs are co-administered with fluvoxamine. The dosage of these benzodiazepines should be reduced during co-administration with fluvoxamine.
Glucuronidation
Fluvoxamine does not affect plasma digoxin concentrations.
Renal excretion
Fluvoxamine does not affect the plasma concentrations of atenolol.
Pharmacodynamic interactions
The serotonergic effects of fluvoxamine may be enhanced when it is used in combination with other serotonergic agents (including tramadol, triptans, linezolid, SSRIs and St. John's wort preparations) (see also section 4.4).
Fluvoxamine has been used in combination with lithium in the treatment of severely ill, treatment-resistant patients. However lithium (and possibly also tryptophan) increases the serotonergic effect of fluvoxamine. Caution should therefore be exercised in using this combination in patients with severe, treatment-resistant depression.
In patients taking oral anticoagulants and fluvoxamine, the risk of bleeding may be increased and therefore these patients should be monitored closely.
As with other psychotropic drugs, patients should be advised not to take alcohol while on fluvoxamine.
04.6 Pregnancy and lactation
Pregnancy
Epidemiological data have suggested that the use of Selective Serotonin Reuptake Inhibitor (SSRI) drugs during pregnancy, particularly late pregnancy, may increase the risk of persistent pulmonary hypertension in the newborn (PPHN). The observed risk was approximately 5 cases per 1000 pregnancies. In the general population, 1 to 2 cases of PPHN occur per 1000 pregnancies.
Reproductive toxicity studies in animals have shown an increase in embryotoxicity (embryofoetal death, fetal ocular abnormalities) associated with treatment. The effect in humans is unknown. The safety margin for reproductive toxicity is unknown (see section 5.3). FEVARIN should not be used during pregnancy unless the clinical condition of the patient requires treatment with fluvoxamine.
There have been isolated reports of withdrawal symptoms in neonates following the use of fluvoxamine at the end of pregnancy.
Some infants exposed to SSRIs in the last trimester of pregnancy have shown difficulty feeding and / or breathing difficulties, convulsions, unstable temperature, hypoglycemia, tremor, abnormal muscle tone, nervousness, cyanosis, irritability, lethargy, drowsiness, vomiting, difficulty in constant sleep and crying and prolongation of hospitalization may be necessary.
Feeding time
Fluvoxamine is excreted in breast milk in small quantities. Therefore the drug should not be given to women who are breastfeeding.
Fertility
Reproductive toxicity studies in animals have shown that FEVARIN adversely affects male and female fertility. The safety margin for this effect has not been identified and its relevance to humans is unknown.
Animal data have shown that fluvoxamine can affect sperm quality (see section 5.3).
In humans, reports from patients treated with SSRIs have shown that the effect on sperm quality is reversible.
No impact on fertility has been observed so far.
FEVARIN should not be used in patients seeking conception unless their clinical condition requires treatment with fluvoxamine.
04.7 Effects on ability to drive and use machines
Fluvoxamine up to 150 mg has no or negligible influence on the ability to drive and use machines. It has been shown in healthy volunteers to have no effect on the psychomotor skills required to drive and use machines. However, somnolence has been reported during treatment with fluvoxamine. Therefore, caution is recommended until the individual response to the drug is ascertained.
04.8 Undesirable effects
Adverse events, observed in clinical trials at the frequency described below, are often associated with the disease and are not necessarily related to treatment.
Frequency estimate: very common (≥ 1/10), common (≥ 1/100,
Nausea, sometimes associated with vomiting, is the most frequently observed symptom associated with fluvoxamine treatment. This side effect usually subsides within the first two weeks of treatment.
** Class Effects: Epidemiological studies, mainly conducted in patients aged 50 years or older, show an increased risk of bone fractures in patients treated with Selective Serotonin Reuptake Inhibitors (SSRIs) and tricyclic antidepressants (TCAs). The mechanism leading to this risk is not known.
Cases of suicidal ideation and suicidal behaviors have been observed during fluvoxamine therapy or shortly after treatment discontinuation (see section 4.4 Special warnings and precautions for use).
Withdrawal symptoms observed after discontinuation of fluvoxamine
Discontinuation symptoms are common following discontinuation of fluvoxamine (especially if abrupt).
Dizziness, sensory disturbances (including paraesthesia, visual disturbances, electric shock sensations), sleep disturbances (including insomnia and intense dreams), agitation and anxiety, irritability, confusion, emotional instability, nausea and / or vomiting, diarrhea, sweating, palpitations , headache and tremor are the most commonly reported reactions. In general, these symptoms are mild to moderate in intensity and are self-limiting, although in some patients they may be severe and / or prolonged. It is therefore recommended that, when treatment with fluvoxamine is no longer required, gradual discontinuation by reducing the dose (see sections 4.2 and 4.4).
Pediatric population
In a 10-week placebo-controlled study in children and adolescents with OCD, adverse events frequently reported with an incidence higher than placebo were: insomnia, asthenia, agitation, hyperkinesia, somnolence and dyspepsia. Adverse events serious in this study included: agitation and hypomania.
Convulsions have been observed in children and adolescents while using the drug outside of clinical trials.
04.9 Overdose
Symptoms
Symptoms include gastrointestinal disturbances (nausea, vomiting, diarrhea), sleepiness and dizziness. Cardiac events (tachycardia, bradycardia, hypotension), abnormal liver function, convulsions and coma have also been reported.
Fluvoxamine has a large margin of safety in the event of an overdose. Since marketing, reports of death attributed to an overdose of fluvoxamine alone have been extremely rare. The highest documented dose of fluvoxamine ingested by a patient is 12 grams. This patient has recovered completely. Occasionally more serious complications have been observed. in case of deliberate overdose of fluvoxamine in combination with other drugs.
Treatment
There is no specific antidote to fluvoxamine available.
In the event of an overdose, it is advisable to proceed as soon as possible after ingestion of the stomach-emptying tablets and to institute symptomatic treatment. The repeated use of medicinal charcoal is also recommended, if necessary accompanied by an osmotic laxative.
Forced diuresis or dialysis is unlikely to be effective.
05.0 PHARMACOLOGICAL PROPERTIES
05.1 Pharmacodynamic properties
Pharmacotherapeutic group: antidepressants, selective serotonin reuptake inhibitors.
ATC code: N06AB08.
The mechanism of action of fluvoxamine is thought to be related to the selective inhibition of serotonin reuptake at the level of brain neurons. It has only modest interference with noradrenergic processes. Receptor binding studies have shown that fluvoxamine has negligible affinity for alpha-adrenergic, beta-adrenergic, histaminergic, muscarinic, dopaminergic and serotonergic receptors.
In a placebo-controlled study of 120 OCD patients aged 8 to 17 years, a statistically significant improvement in the total population was observed in favor of fluvoxamine at week 10. A further subgroup analysis showed improvement on the C-YBOCS scale in children while no effect was observed in adolescents. The mean dose was 158 and 168 mg / day, respectively.
Dose / response
No formal clinical studies have been conducted to establish the dose / response relationship of fluvoxamine. However, clinical experience shows that upward titration of the dose may be beneficial in some patients.
05.2 Pharmacokinetic properties
Absorption
Fluvoxamine is completely absorbed following oral administration. The maximum plasma concentration occurs within 3-8 hours after administration. The mean absolute bioavailability is 53%, due to first pass metabolism.
The pharmacokinetics of fluvoxamine are not affected by concomitant food intake.
Distribution
In vitro, plasma protein binding is 80%. The volume of distribution in humans is 25 l / kg.
Metabolism
Fluvoxamine undergoes extensive hepatic metabolism. Although CYP2D6 is the major isoenzyme involved in the metabolism of fluvoxamine in vitro, plasma concentrations of fluvoxamine in poor metabolisers are not much higher than those in extensive metabolisers.
The mean plasma half-life is approximately 13-15 hours after single administration and slightly longer (17-22 hours) after repeated administration, while steady-state is generally achieved within 10-14 days.
Fluvoxamine is extensively transformed in the liver, mainly through oxidative demethylation, with the formation of at least nine renally eliminated metabolites. The two main metabolites showed negligible pharmacological activity. The other metabolites are not expected to be pharmacologically active. Fluvoxamine is a potent inhibitor of CYP1A2 and a moderate inhibitor of CYP2C and CYP3A4, with only marginal inhibitory effects on CYP2D6. Fluvoxamine exhibits linear pharmacokinetics following a single dose. Steady-state concentrations are higher than those calculated after single dose and, at higher daily doses, are disproportionately higher.
Special patient groups
The pharmacokinetics of fluvoxamine are similar in healthy adults, the elderly and patients with renal insufficiency. The metabolism of fluvoxamine is impaired in patients with liver disease.
Steady-state plasma concentrations of fluvoxamine are twice as high in children (aged 6 to 11 years) as in adolescents (ages 12-17). Plasma concentrations in adolescents are similar to those in adults.
05.3 Preclinical safety data
Carcinogenesis and mutagenesis
There is no evidence of carcinogenic or mutagenic effects with fluvoxamine.
Fertility and reproductive toxicity
Studies on male and female animal fertility have shown decreased performance during mating, decreased sperm count and fertility index, and increased ovarian weight at levels above human exposure.
Reproductive toxicity studies in rats showed that fluvoxamine is embryotoxic (increased embryofetal deaths [resorptions], increased ocular fetal abnormalities [folded retina], decreased fetal weight and delayed ossification). Effects on weight of the fetus and ossification are likely secondary to maternal toxicity (decreased maternal body weight and weight gain).
In addition, an "increased incidence of perinatal mortality in puppies was observed in pre- and postnatal studies."
The safety margin for reproductive toxicity is unknown.
Physical and psychological dependence
The potential for the establishment of abuse, tolerance and physical dependence has been studied in non-human primate models. No addiction phenomena have been highlighted.
06.0 PHARMACEUTICAL INFORMATION
06.1 Excipients
Nucleus
Mannitol, corn starch, pregelatinised starch, sodium stearyl fumarate, anhydrous colloidal silica.
Coating
Hypromellose, macrogol 6000, talc, titanium dioxide E171.
06.2 Incompatibility
Not relevant.
06.3 Period of validity
3 years.
06.4 Special precautions for storage
Do not store above 25 ° C.
06.5 Nature of the immediate packaging and contents of the package
PVC / PVDC / aluminum blisters
Packs of 5, 10, 20, 30, 50, 60, 90, 100 and 250 tablets.
Not all pack sizes may be marketed.
06.6 Instructions for use and handling
No special instructions.
07.0 MARKETING AUTHORIZATION HOLDER
BGP PRODUCTS B.V WEGALAAN 9 HOOFDDORP (HOLLAND)
08.0 MARKETING AUTHORIZATION NUMBER
FEVARIN 50 mg film-coated tablets, 30 tablets, AIC No. 027045032
09.0 DATE OF FIRST AUTHORIZATION OR RENEWAL OF THE AUTHORIZATION
24.05.90 / 21.06.2009