Active ingredients: Escin, Diethylaminasalicylate
Liotontrauma 2% + 5% gel
Indications Why is Liotontrauma used? What is it for?
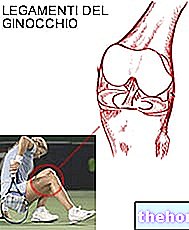
Liotontrauma is a cutaneous (skin) medicine containing the active substances escin and diethylaminasalicylate used to treat joint and muscle pain following trauma (minor trauma).
Contraindications When Liotontrauma should not be used
Do not take Liotontrauma
- if you are allergic to aescin and diethylaminasalicylate or any of the other ingredients of this medicine (listed in section 6)
Do not use Liotontrauma on open lesions (wounds), mucous membranes and areas of skin treated with radiation
Precautions for use What you need to know before taking Liotontrauma
Talk to your doctor or pharmacist before using Liotontrauma.
There is no risk of addiction (decreased effectiveness) and dependence (need to continue taking the medicine longer than necessary).
Being a preparation for local applications, its use must be exclusively external (on the skin only).
The prolonged use of products for cutaneous use can give rise to allergy phenomena (sensitization).
Interactions Which drugs or foods can modify the effect of Liotontrauma
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
No interaction with other medicinal products is known.
This medicine should not be applied together with other products.
Warnings It is important to know that:
Pregnancy and breastfeeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
In pregnancy and / or breastfeeding Liotontrauma should only be used after consultation with and under close medical supervision. However, prolonged use (maximum 3 weeks) of the medicine on large areas of the skin during pregnancy and use on the breast during breastfeeding should be avoided.
Driving and using machines
Liotontrauma does not affect the ability to drive or use machines.
Dose, Method and Time of Administration How to use Liotontrauma: Posology
Always use this medicine exactly as described in this leaflet or as directed by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist.
Adults and adolescents (12-18 years)
Apply Liotontrauma 1 to 3 times a day on the area to be treated.
The quantity to be applied depends on the extension of the area to be treated.
Apply a thin layer of Liotontrauma directly to the skin of the area to be treated. After each application, wash your hands thoroughly.
Warning: do not exceed the indicated doses and use only for short periods of treatment.
Consult your doctor if the disorder occurs repeatedly or if you have noticed any recent changes in its characteristics
Overdose What to do if you have taken too much Liotontrauma
If you use more Liotontrauma than you should
No cases of overdose have been reported.
If an overdose of Liotontrauma has been applied, wash the affected area thoroughly.
In case of accidental ingestion / intake of an excessive dose of Liotontrauma, notify your doctor immediately or go to the nearest hospital.
If you stop taking Liotontrauma
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
Side Effects What are the side effects of Liotontrauma
Like all medicines, this medicine can cause side effects, although not everybody gets them.
In rare cases, allergies (hypersensitivity reactions) such as redness, peeling and dryness (dehydration) of the skin may occur.
Compliance with the instructions contained in the package leaflet reduces the risk of undesirable effects.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system at: https://www.aifa.gov.it/content/segnalazioni-reazioni-avverse
By reporting side effects you can help provide more information on the safety of this medicine.
Expiry and Retention
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton after "EXP".
The expiry date refers to the last day of that month.
Store at a temperature not exceeding 30 ° C.
Do not throw any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. This will help protect the environment.
It is important to always have the information on the medicine available, so keep both the box and the package leaflet.
What Liotontrauma 2% + 5% contains
100 g of gel contains 2 g of escin and 5 g diethylaminasalicylate.
The other ingredients are:
lavender essence, nerolene essence, carboxypolymethylene, meglumine, propylene glycol, ethyl alcohol, sodium edetate, hexyldecanol and hexyldecyl laurate, ethoxydiglycol, butylhydroxytoluene, titanium dioxide, purified water
Description of Liotontrauma's appearance and contents of the pack
Liotontrauma 2% + 5% comes in the form of a gel for cutaneous use.
The contents of the package is a tube of 40 g of gel.
Source Package Leaflet: AIFA (Italian Medicines Agency). Content published in January 2016. The information present may not be up-to-date.
To have access to the most up-to-date version, it is advisable to access the AIFA (Italian Medicines Agency) website. Disclaimer and useful information.
01.0 NAME OF THE MEDICINAL PRODUCT
LIOTONTRAUMA 2% + 5% GEL
02.0 QUALITATIVE AND QUANTITATIVE COMPOSITION
100 g of gel contain: Aescin 2 g
Diethylamine salicylate 5 g
For the full list of excipients, see 6.1.
03.0 PHARMACEUTICAL FORM
Gel.
04.0 CLINICAL INFORMATION
04.1 Therapeutic indications
Minor traumatology.
04.2 Posology and method of administration
Apply and spread a thin layer of LIOTONTRAUMA gel on the skin of the area to be treated 1 to 3 times a day.
04.3 Contraindications
Hypersensitivity to the active substances or to any of the excipients listed in section 6.1.
LIOTONTRAUMA gel should not be used on open lesions (wounds), mucous membranes and skin areas treated with radiation.
04.4 Special warnings and appropriate precautions for use
There are no risks of addiction and dependence.
Being a preparation for topical applications, its use must be exclusively external. The use, especially prolonged, of products for topical use can give rise to sensitization phenomena.
04.5 Interactions with other medicinal products and other forms of interaction
No interactions with other medicinal products have been reported.
04.6 Pregnancy and lactation
In case of pregnancy and while breastfeeding it is not recommended to use LIOTONTRAUMA gel unless under close medical supervision. However, it is advisable to avoid prolonged use (maximum 3 weeks) of the product on large skin areas during pregnancy and use on the breast during breastfeeding.
04.7 Effects on ability to drive and use machines
LIOTONTRAUMA gel does not affect the ability to drive or use machines.
04.8 Undesirable effects
In rare cases, hypersensitivity reactions such as redness, peeling and dehydration of the skin may appear.
Reporting of suspected adverse reactions
Reporting of suspected adverse reactions occurring after authorization of the medicinal product is important as it allows continuous monitoring of the benefit / risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system. "address: www.agenziafarmaco.gov.it/it/responsabili.
04.9 Overdose
No cases of overdose have been reported.
05.0 PHARMACOLOGICAL PROPERTIES
05.1 Pharmacodynamic properties
Pharmacotherapeutic group: drugs for topical use, for joint and muscle pain. ATC code: M02AC.
Escin acts on the vascular walls. In case of increased permeability due to inflammation it reduces exudation, limiting the extravasation of liquids into the tissue and accelerating the absorption of the existing edema. The mechanism of action is based on the modification of the permeability of the affected capillary openings. Furthermore, escin increases the resistance of the capillaries, has an anti-inflammatory effect and improves microcirculation.
Diethylamine salicylate has remarkable analgesic properties. It is easily absorbed by the skin and develops its analgesic action in depth on the treated area. The anti-inflammatory action of diethylamine salicylate reinforces the anti-inflammatory action of escin, eliminating the causes of the disease.
05.2 "Pharmacokinetic properties
It has been shown, in various animal species and in humans, that the absorption of aescin after topical application is very low (
At the point of application, concentrations are clearly measurable in the subcutaneous area and in the underlying musculature. Aescin is not detectable in human blood and urine.
According to the experiments carried out on animals and the literature available on the subject, salicylates are more absorbed. However, the values found in the blood after topical treatment for therapeutic purposes do not fall within the toxicity range.
05.3 Preclinical safety data
Non-clinical data reveal no risk for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity, reproductive toxicity.
06.0 PHARMACEUTICAL INFORMATION
06.1 Excipients
Lavender essence, nerolene essence, carboxypolymethylene, meglumine, propylene glycol, ethyl alcohol, sodium edetate, hexyldecanol and hexyldecyl laurate, ethoxydiglycol, butylhydroxytoluene, titanium dioxide, purified water.
06.2 Incompatibility
Not relevant.
06.3 Period of validity
3 years.
06.4 Special precautions for storage
Store at a temperature not exceeding 30 ° C.
06.5 Nature of the immediate packaging and contents of the package
40 g aluminum tube with internal protective layer and screw cap.
06.6 Instructions for use and handling
No special instructions.
Unused product and waste derived from this medicine must be disposed of in accordance with local legal requirements.
07.0 MARKETING AUTHORIZATION HOLDER
Sanofi S.p.A.
Viale L. Bodio 37 / B - IT - 20158 MILAN
08.0 MARKETING AUTHORIZATION NUMBER
LIOTONTRAUMA 2% + 5% gel, tube 40 g A.I.C. 037375021
09.0 DATE OF FIRST AUTHORIZATION OR RENEWAL OF THE AUTHORIZATION
Date of first authorization: 17.12.2007