Active ingredients: Candesartan
Atacand 4 mg tablets
Atacand 8 mg tablets
Atacand 16 mg tablets
Atacand 32 mg tablets
Why is Atacand used? What is it for?
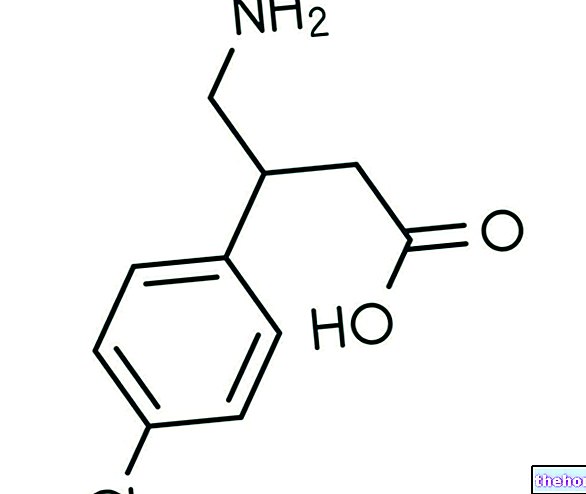
The name of the medicine is Atacand. The active ingredient is candesartan cilexetil. It belongs to a group of medicines called angiotensin II receptor antagonists. It works by causing blood vessels to relax and widen. This helps to lower blood pressure. It also makes the heart pump blood more easily around the world. body.
Atacand can be used for:
- treat high blood pressure (hypertension) in adult patients and in children and adolescents aged 6 to 18 years.
- treat heart failure in adult patients with impaired heart muscle function when Angiotensin Converting Enzyme (ACE) inhibitors cannot be used or in addition to ACE inhibitors when symptoms persist despite treatment and antagonists mineralocorticoid receptor (MRA) cannot be used (ACE inhibitors and MRAs are medicines used to treat heart failure).
Contraindications When Atacand should not be used
Do not take Atacand:
- if you are allergic to candesartan cilexetil or any of the other ingredients of this medicine (listed in section 6).
- if you are more than three months pregnant (it is also better to avoid Atacand in early pregnancy - see pregnancy section)
- if you have severe liver disease or biliary obstruction (a problem with the drainage of bile from the gallbladder)
- if the patient is a child under 1 year of age. if he has diabetes or impaired kidney function and is being treated with a blood pressure lowering medicine containing aliskiren.
If you are not sure if any of these apply to you, consult your doctor or pharmacist before taking Atacand.
Precautions for use What you need to know before taking Atacand
Talk to your doctor before taking Atacand:
- if you have heart, liver or kidney problems or are on dialysis.
- if you have recently had a kidney transplant.
- if you are vomiting, have recently had severe vomiting or have diarrhea.
- if you have a disease of the adrenal gland known as Conn's syndrome (also called primary aldosteronism).
- if you have low blood pressure.
- if you have ever had a stroke.
- tell your doctor if you think you are pregnant (or if there is a possibility of becoming pregnant). Atacand is not recommended in early pregnancy, and must not be taken if you are more than three months pregnant, as it may cause serious harm to your baby if used at that stage (see pregnancy section).
- if you are taking any of the following medicines used to treat high blood pressure:
- an "ACE inhibitor" (eg enalapril, lisinopril, ramipril), particularly if you have diabetes-related kidney problems.
- aliskiren.
- If you are taking an ACE inhibitor together with a medicine belonging to the class of medicines known as mineralocorticoid receptor antagonists (MRAs). These medicines are used to treat heart failure (see "Taking other medicines")
Your doctor may check your kidney function, blood pressure and the amount of electrolytes (for example potassium) in your blood at regular intervals.
See also information under the heading "Do not take Atacand if".
Your doctor may need to see you more often and get tested if you have any of these conditions.
If you are about to have an operation, tell your doctor or dentist that you are taking Atacand. This is because Atacand, when combined with some anesthetic, can cause a drop in blood pressure.
Children and adolescents
Atacand has been studied in children. For more information, ask your doctor. Atacand should not be given to children under 1 year of age due to the potential risk to developing kidneys.
Interactions Which drugs or foods can modify the effect of Atacand
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines. Atacand can affect the way some other medicines work, and some medicines can have an effect on Atacand. If you are taking certain medicines, your doctor may need to have blood tests from time to time.
In particular, tell your doctor if you are taking any of the following medicines as your doctor may need to change your dose and / or take other precautions:
- other medicines that help lower blood pressure, including beta blockers, diazoxide and ACE inhibitors such as enalapril, captopril, lisinopril or ramipril
- non-steroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen, naproxen, diclofenac, celecoxib, or etoricoxib (medicines to relieve pain and inflammation)
- acetylsalicylic acid (if you are taking more than 3 g per day) (medicine to relieve pain and inflammation)
- potassium supplements or potassium-containing salt substitutes (medicines that increase the level of potassium in the blood)
- heparin (a medicine to thin the blood)
- cotrimoxazole (an antibiotic) also known as trimethoprim / sulfamethoxazole.
- tablets that help you urinate (diuretics)
- lithium (a medicine for mental health problems)
- if you are taking an ACE inhibitor or aliskiren (see also information under the headings: "Do not take Atacand if" and "Take special care with Atacand")
- if you are being treated with an ACE inhibitor together with other medicines used for heart failure, known as mineralocorticoid receptor antagonists (MRA) (eg spironolactone, eplerenone).
Atacand with food, drink and alcohol
- You can take Atacand with or without food.
- When prescribed Atacand, talk to your doctor before drinking alcohol. Alcohol can make you feel faint or lightheaded.
Warnings It is important to know that:
Pregnancy and breastfeeding
Pregnancy
You should tell your doctor if you think you are pregnant (or if there is a possibility of becoming pregnant). Your doctor will usually advise you to stop taking Atacand before becoming pregnant or as soon as you know you are pregnant and will advise you to take another medicine instead of Atacand. pregnancy, and must not be taken if you are more than three months pregnant, as it may cause serious harm to your baby if taken after the third month of pregnancy.
Feeding time
Tell your doctor if you are breastfeeding or about to start breastfeeding. Atacand is not recommended for women who are breastfeeding and your doctor may choose another treatment for you if you wish to breastfeed, especially if your baby is newborn or born. premature.
Driving and using machines
Some people may feel tired or lightheaded when taking Atacand. If this happens to you, do not drive or use any tools or machines.
Atacand contains lactose. Lactose is a type of sugar. If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicinal product.
Dose, Method and Time of Administration How to use Atacand: Posology
Always take this medicine exactly as your doctor has told you. If in doubt, consult your doctor or pharmacist. It is important to keep taking Atacand every day. Atacand can be taken with or without food.
Swallow the tablet with a drink of water.
Try to take the tablet at the same time each day. This will help you remember to take it.
High blood pressure:
- The usual dose of Atacand is 8 mg once a day. Your doctor may increase this dose up to 16 mg once a day and further up to 32 mg once a day depending on your blood pressure response.
- In some patients, such as those with liver problems, kidney problems or who have recently lost fluids due to for example: vomiting, diarrhea or taking tablets that help urinate, the doctor may prescribe a lower starting dosage.
- Some black patients may respond poorly to this medicine when given as the only treatment and may need a higher dose.
Use in children and adolescents with high blood pressure
Children aged 6 to 18:
The recommended starting dose is 4 mg once a day.
For patients weighing less than 50 kg: In some patients whose blood pressure is not adequately controlled, the doctor may decide whether the dose should be increased up to a maximum of 8 mg once a day.
For patients weighing 50 kg or more: In some patients whose blood pressure is not adequately controlled, the doctor may decide whether the dose should be increased to 8 mg once daily and 16 mg once daily.
Heart failure in adults:
- the recommended starting dose of Atacand is 4 mg once a day. Your doctor may increase this dosage by doubling the dose at intervals of at least 2 weeks to 32 mg once a day. Atacand can be taken together with other medicines for heart failure, and your doctor will decide which treatment is best for you.
If you forget to take Atacand
Do not take a double dose to make up for a forgotten tablet. Just take the next dose as usual.
If you stop taking Atacand
If you stop taking Atacand, your blood pressure may rise again.
Therefore do not stop taking Atacand without first talking to your doctor.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
Overdose What to do if you have taken too much Atacand
If you take more Atacand than prescribed by your doctor, contact a doctor or pharmacist immediately for advice.
Side Effects What are the side effects of Atacand
Like all medicines, this medicine can cause side effects, although not everybody gets them. It is important that you are aware of what these side effects may be.
Stop taking Atacand and seek medical help immediately if you experience any of the following allergic reactions:
- difficulty in breathing, with or without swelling of the face, lips, tongue and / or throat
- swelling of your face, lips, tongue and / or throat, which may cause you difficulty in swallowing
- severe itchy skin (with raised blisters)
Atacand can cause a reduction in the number of white blood cells in the blood. Your resistance to infection may decrease and you may notice tiredness, infection or fever. If this happens, contact your doctor. Your doctor may occasionally perform blood tests to check if Atacand has had any effects on your blood (agranulocytosis).
Possible side effects include:
Common (affects 1 to 10 users in 100)
- Feeling dizzy / lightheaded
- Headache
- Respiratory infection
- Low blood pressure. This can make you feel faint or lightheaded.
- Changes in blood test results:
- An increased amount of potassium in the blood, especially if you already have kidney problems or heart failure. If this is severe then you may also notice tiredness, weakness, an irregular heartbeat or tingling.
- Effects on how your kidneys work, especially if you already have kidney problems or heart failure. In very rare cases, kidney failure can occur.
Very rare (affects less than 1 user in 10,000)
- Swelling of the face, lips, tongue and / or throat.
- A reduction in red or white blood cells. You may notice tiredness, an infection or a fever.
- Skin rash, swollen rash (hives).
- Itching.
- Back pain, pain in the joints and muscles.
- Changes in the way the liver works, including inflammation of the liver (hepatitis). You may notice tiredness, yellowing of the skin and whites of the eyes and flu-like symptoms.
- Nausea.
- Changes in blood test results:
- A low amount of sodium in the blood. If this is severe then you may also notice weakness, lack of energy or muscle cramps.
- Cough.
In children treated for high blood pressure, side effects appear to be similar to those seen in adults, but they occur more often. Sore throat in children is a very common but not reported side effect in adults and rhinorrhea (runny nose), fever and increased heart rate are common in children but not reported in adults.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system at www.agenziafarmaco.gov. it / it / responsible. By reporting side effects you can help provide more information on the safety of this medicine.
Expiry and Retention
- Keep this medicine out of the sight and reach of children
- Do not use this medicine after the expiry date which is stated on the carton or blister. The expiry date refers to the last day of the month.
- This medicine does not require any storage temperatures.
- Do not throw any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. This will help protect the environment.
Deadline "> Other information
What Atacand contains
The active ingredient is candesartan cilexetil. The tablets contain, 4 mg, 8 mg, 16 mg or 32 mg of candesartan cilexetil.
The other ingredients are calcium carmellose, hydroxypropylcellulose, lactose monohydrate, magnesium stearate, maize starch, macrogol. The 8 mg, 16 mg and 32 mg tablets also contain iron oxide (E172).
Description of what Atacand looks like and contents of the pack
- The 4 mg tablets are white, round with a score line and debossed with A / CF on one side and 004 on the other.
- The 8 mg tablets are pale pink, round with a score line and debossed with A / CG on one side and 008 on the other.
- The 16 mg tablets are pink, round with a score line and debossed with A / CH on one side and 016 on the other.
- The 32 mg tablets are pink, round with a score line and debossed with A / CL on one side and 032 on the other.
The tablet can be divided into equal halves by cutting along the score line.
The tablets are presented in plastic bottles containing 100 or 250 tablets or in blisters containing 7, 14, 15, 15x1, 20, 28, 30, 30x1, 50, 50x1, 56, 90, 98, 98x1, 100 or 300 tablets.
Not all pack sizes may be marketed in all countries.
Source Package Leaflet: AIFA (Italian Medicines Agency). Content published in January 2016. The information present may not be up-to-date.
To have access to the most up-to-date version, it is advisable to access the AIFA (Italian Medicines Agency) website. Disclaimer and useful information.
01.0 NAME OF THE MEDICINAL PRODUCT -
RATACAND TABLETS
02.0 QUALITATIVE AND QUANTITATIVE COMPOSITION -
4 mg: each tablet contains 4 mg of candesartan cilexetil.
8 mg: each tablet contains 8 mg of candesartan cilexetil.
16 mg: each tablet contains 16 mg of candesartan cilexetil.
32 mg: each tablet contains 32 mg of candesartan cilexetil.
Excipients:
4 mg: each tablet contains 93.4 mg of lactose monohydrate.
8 mg: each tablet contains 89.4 mg of lactose monohydrate.
16 mg: each tablet contains 81.4 mg of lactose monohydrate.
32 mg: each tablet contains 163 mg of lactose monohydrate.
For the full list of excipients, see section 6.1.
03.0 PHARMACEUTICAL FORM -
Tablet.
4 mg: white, round tablets (diameter 7 mm) with a score line and embossed with A / CF on one side and 004 on the other.
8 mg: light pink, round tablets (diameter 7 mm) with a score line and embossed with A / CG on one side and 008 on the other.
16 mg: pink, round tablets (diameter 7 mm) with a score line and embossed with A / CH on one side and 016 on the other.
32 mg: pink, round tablets (9.5 mm diameter) with a score line and embossed with A / CL on one side and 032 on the other.
The tablet can be divided into equal halves.
04.0 CLINICAL INFORMATION -
04.1 Therapeutic indications -
Atacand is indicated for:
Treatment of essential hypertension in adults.
Treatment of adult patients with heart failure and impaired left ventricular systolic function (left ventricular ejection fraction ≤ 40%) in addition to treatment with Angiotensin Converting Enzyme (ACE) inhibitors or when ACE inhibitors are not tolerated ( see paragraph 5.1).
04.2 Posology and method of administration -
Dosage in hypertension
The recommended starting dose and the usual maintenance dose of Atacand is 8 mg once a day. Most of the antihypertensive effect is achieved within 4 weeks. In some patients whose blood pressure is not adequately controlled, the dose may be increased up to 16 mg once daily and up to a maximum of 32 mg once daily. day. Therapy should be adapted according to blood pressure response. Atacand can also be administered with other antihypertensive agents. The addition of hydrochlorothiazide has shown an additional antihypertensive effect with various doses of Atacand.
Elderly population
No initial dosage adjustment is necessary in elderly patients.
Patients with intravascular volume depletion
In patients at risk of hypotension, such as patients with possible intravascular volume depletion, a starting dose of 4 mg may be considered (see section 4.4).
Patients with impaired renal function
In patients with impaired renal function the starting dose is 4 mg, including patients on hemodialysis. The dose should be titrated based on the response. Experience in patients with very severe or end-stage renal insufficiency (Clcreatinine
Patients with impaired hepatic function
A starting dose of 4 mg once daily is recommended in patients with mild and moderate hepatic impairment. The dose can be adjusted based on the response. Atacand is contraindicated in patients with severe hepatic impairment and / or cholestasis (see sections 4.3 and 5.2).
Black patients
The antihypertensive effect of candesartan is less pronounced in black patients than in non-black patients. Therefore, increased doses of Atacand and the addition of concomitant therapy may be more frequently needed for blood pressure control in patients. Black versus non-blacks (see section 5.1).
Dosage in Heart Failure
The usual recommended starting dose of Atacand is 4 mg once daily. Titration to the target dose of 32 mg once daily (maximum dose) or to the highest tolerated dose is done by doubling the dose at intervals of at least 2 weeks (see section 4.4). Evaluation of patients with heart failure should always include monitoring of renal function, including serum creatinine and potassium.
Atacand can be given with other treatments for heart failure, including ACE inhibitors, beta-blockers, diuretics and digitalis or a combination of these medicines. The combination of an ACE inhibitor, a potassium-sparing diuretic (e.g. spironolactone) and Atacand is not recommended and should only be considered after careful consideration of the potential benefits and risks (see sections 4.4, 4.8 and 5.1).
Special patient populations
No initial dosage adjustment is necessary in elderly patients or in patients with intravascular volume depletion, renal impairment or mild to moderate hepatic impairment.
Pediatric population
The safety and efficacy of Atacand in children from birth to 18 years have not been established in the treatment of hypertension and heart failure. No data are available.
Method of administration
Oral use
Atacand should be administered once daily with or without food.
The bioavailability of candesartan is not affected by food.
04.3 Contraindications -
Hypersensitivity to candesartan cilexetil or to any of the excipients.
Second and third trimester of pregnancy (see sections 4.4 and 4.6).
Severe hepatic insufficiency and / or cholestasis.
04.4 Special warnings and appropriate precautions for use -
Altered kidney function
As with other agents that inhibit the renin-angiotensin-aldosterone system, changes in renal function can be expected in susceptible patients treated with Atacand.
Periodic monitoring of serum potassium and creatinine levels is recommended when Atacand is used in hypertensive patients with impaired renal function. Experience is limited in patients with very severe or end-stage renal impairment (Clcreatinine blood pressure monitoring.
Evaluation of patients with heart failure should include periodic assessments of renal function, particularly in elderly patients 75 years of age or older, and in patients with impaired renal function. During dose titration of Atacand, it is recommended to monitor serum creatinine and potassium concentrations. Clinical trials in heart failure did not include patients with serum creatinine> 265 μmol / L (> 3 mg / dL).
Concomitant therapy with ACE inhibitors in heart failure
The risk of adverse reactions, in particular impaired renal function and hyperkalaemia, may be increased when Atacand is given in combination with an ACE inhibitor (see section 4.8). Patients undergoing this treatment should be regularly and carefully monitored.
Hemodialysis
During dialysis blood pressure can be particularly sensitive to AT1 receptor blockade as a result of reduced plasma volume and activation of the renin-angiotensin-aldosterone system. Therefore Atacand should be carefully dosed by monitoring blood pressure in hemodialysis patients.
Renal artery stenosis
Medicinal products that affect the renin-angiotensin-aldosterone system, including angiotensin II receptor antagonists (AIIRAs), may increase blood urea nitrogen and creatinine in patients with bilateral renal artery stenosis or renal artery stenosis in the presence of of single kidney.
Kidney transplant
There is no experience with the use of Atacand in patients who have undergone a recent kidney transplant.
Hypotension
Hypotension may occur during treatment with Atacand in patients with heart failure. This can also occur in hypertensive patients with intravascular volume depletion such as those taking high-dose diuretics. Caution should be used when initiating therapy and attempts should be made to correct hypovolaemia.
Anesthesia and surgery
Hypotension due to blockade of the renin-angiotensin system may occur during anesthesia and surgery in patients treated with angiotensin II antagonists. Very rarely, hypotension can be so severe as to justify the use of intravenous fluids and / or vasopressor substances.
Aortic and mitral stenosis (obstructive hypertrophic cardiomyopathy)
As with other vasodilators, special caution is recommended in patients with haemodynamically relevant aortic or mitral stenosis, or hypertrophic obstructive cardiomyopathy.
Primary hyperaldosteronism
Patients with primary aldosteronism generally do not respond to antihypertensive medicinal products which work by inhibiting the renin-angiotensin-aldosterone system. Therefore the use of Atacand is not recommended in this population.
Hyperkalaemia
Concomitant use of Atacand with potassium-sparing diuretics, potassium supplements, potassium-containing salt substitutes, or other medicinal products that may increase potassium (such as heparin) may cause serum potassium increases in hypertensive patients. serum potassium levels should be done where appropriate.
In heart failure patients treated with Atacand, hyperkalaemia may occur. Periodic monitoring of serum potassium levels is recommended. The combination of an ACE inhibitor, a potassium-sparing diuretic (e.g. spironolactone) and Atacand is not recommended and should only be considered after careful consideration of the potential benefits and risks.
General aspects
In patients whose vascular tone and renal function are predominantly dependent on the activity of the renin-angiotensin-aldosterone system (eg patients with severe congestive heart failure or with underlying renal disease including renal artery stenosis), treatment it has been associated with acute hypotension, azotaemia, oliguria or, rarely, acute renal failure with other medicinal products affecting this system. The possibility of similar effects cannot be excluded with the use of AIIRAs. As with other antihypertensive drugs, the excessive decrease in blood pressure in patients with ischemic heart disease or ischemic cerebrovascular disease can lead to myocardial infarction or stroke.
The antihypertensive effect of candesartan may be enhanced by other medicinal products with hypotensive properties, whether prescribed as antihypertensive or for other indications.
Atacand contains lactose. Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption syndrome should not take this medicine.
Pregnancy
Angiotensin II receptor antagonist therapy (AIIRA) should not be initiated during pregnancy.
For patients planning to become pregnant, alternative antihypertensive treatments with a proven safety profile for use in pregnancy should be used, unless continued therapy with an AIIRA is considered essential. When pregnancy is diagnosed, the treatment with AIIRAs should be stopped immediately and, if appropriate, alternative therapy should be started (see sections 4.3 and 4.6).
04.5 Interactions with other medicinal products and other forms of interaction -
Compounds that have been tested in clinical pharmacokinetic studies include hydrochlorothiazide, warfarin, digoxin, oral contraceptives (ethinyl estradiol / levonorgestrel), glibenclamide, nifedipine and enalapril. No clinically relevant pharmacokinetic interactions with other medicinal products have been identified.
Concomitant use of potassium-sparing diuretics, potassium supplements, potassium-containing salt substitutes or other medicinal products (eg heparin) may increase potassium. If appropriate, monitoring of potassium may be considered (see section 4.4).
Reversible increases in serum lithium concentrations and toxic reactions have been reported during concomitant administration of lithium with ACE inhibitors. A similar effect can occur with AIIRAs. The use of candesartan with lithium is not recommended. If the combination proves necessary, careful monitoring of serum lithium levels is recommended.
When AIIRAs are administered simultaneously with non-steroidal anti-inflammatory drugs (NSAIDs) (eg, selective COX-2 inhibitors, acetylsalicylic acid (> 3g / day) and non-selective NSAIDs), "attenuation of the antihypertensive effect" may occur.
As with ACE inhibitors, concomitant use of AIIRAs and NSAIDs may lead to an increased risk of worsening of renal function including possible acute renal failure and increased serum potassium levels, especially in patients with pre-existing impaired renal function. The combination should be administered with caution, especially in the elderly. Patients should be adequately hydrated and monitoring of renal function should be considered at the initiation of concomitant therapy and thereafter periodically.
04.6 Pregnancy and breastfeeding -
Pregnancy
The use of Angiotensin II Receptor Antagonists (AIIRAs) is not recommended during the first trimester of pregnancy (see section 4.4). The use of AIIRAs is contraindicated during the second and third trimesters of pregnancy (see sections 4.3 and 4.4).
Epidemiological evidence on the risk of teratogenicity following exposure to ACE inhibitors during the first trimester of pregnancy has not been conclusive; however a small increase in risk cannot be excluded. Although controlled epidemiological data on the risk of Angiotensin II Receptor Antagonists (AIIRAs) are not available, a similar risk may also exist for this class of medicines. proven safety profile for use in pregnancy unless continued therapy with an AIIRA is considered essential.When pregnancy is diagnosed, treatment with AIIRAs should be stopped immediately and, if appropriate, alternative therapy should be started.
Exposure to AIIRAs during the second and third trimesters is known to induce fetal toxicity (decreased renal function, oligohydramnios, skull ossification retardation) and neonatal toxicity (renal failure, hypotension, hyperkalaemia) in women (see section 5.3). .
Should exposure to AIIRAs have occurred from the second trimester of pregnancy, ultrasound check of renal function and skull is recommended.
Neonates whose mothers have taken AIIRAs should be closely monitored for hypotension (see sections 4.3 and 4.4).
Feeding time
Since no data are available regarding the use of Atacand during breastfeeding, Atacand is not recommended and alternative treatments with a proven safety profile for use during breastfeeding are preferred, especially when nursing a newborn or preterm infant.
04.7 Effects on ability to drive and use machines -
No studies on the effects of candesartan on the ability to drive and use machines have been performed. However, it should be taken into account that occasionally dizziness or fatigue may occur during treatment with Atacand.
04.8 Undesirable effects -
Treatment of hypertension
In controlled clinical trials, adverse reactions were mild and transient. The total incidence of adverse events showed no correlation with dose or age. Discontinuation of treatment due to adverse events was similar with candesartan cilexetil (3.1%) and placebo (3.2%).
From a pooled analysis of data from clinical trials in hypertensive patients, adverse reactions with candesartan cilexetil were defined based on the incidence of adverse events with candesartan cilexetil at least 1% higher than the incidence observed with placebo. Based on this definition, the most commonly reported adverse reactions were dizziness / vertigo, headache and respiratory infections.
The table below presents adverse reactions reported from clinical studies and post-marketing experience.
Frequencies used in the tables throughout section 4.8 are: very common (≥ 1/10), common (≥1 / 100a
Laboratory tests
There were generally no clinically relevant influences of Atacand on routine laboratory parameters. As with other inhibitors of the renin-angiotensin-aldosterone system, slight decreases in hemoglobin have been observed. No routine laboratory monitoring is usually required in patients treated with Atacand. However, in patients with impaired renal function, it is recommended. to periodically check serum potassium and creatinine levels.
Treatment of heart failure
The tolerability profile of Atacand observed in heart failure patients was consistent with drug pharmacology and patients' health status. In the CHARM clinical program, which compared Atacand at doses up to 32 mg (n = 3,803) with placebo (n = 3,796), 21.0% of the candesartan cilexetil group and 16.1% of the placebo group discontinued treatment due to adverse events. The most commonly reported adverse reactions were hyperkalaemia, hypotension, renal impairment.
These events were more common in patients over 70 years of age, diabetics or those who had received other medicinal products that affect the renin-angiotensin-aldosterone system, particularly an ACE inhibitor and / or spironolactone.
The table below presents adverse reactions reported from clinical studies and post-marketing experience.
Laboratory tests
Hyperkalaemia and renal impairment are common in patients treated with Atacand for the indication of heart failure. Periodic monitoring of serum creatinine and potassium concentrations is recommended (see section 4.4).
04.9 Overdose -
Symptoms
Based on pharmacological considerations, the main manifestation of overdose should be symptomatic hypotension and dizziness. In individual reports of overdose (up to 672 mg candesartan cilexetil), patient recovery occurs without consequences.
Methods of intervention in case of overdose
Should symptomatic hypotension occur, symptomatic treatment should be instituted and vital functions monitored. The patient should be placed in the supine position with the legs raised. If this is not sufficient, the plasma volume should be increased by infusion, for example, of isotonic saline. Sympathomimetic medicinal products can be administered if the above measures are insufficient.
Candesartan is not removed by hemodialysis.
05.0 PHARMACOLOGICAL PROPERTIES -
05.1 "Pharmacodynamic properties -
Pharmaco-therapeutic category: angiotensin II antagonists, unassociated.
ATC code: C09CA06.
Angiotensin II is the main vasoactive hormone of the renin-angiotensin-aldosterone system and plays a role in the pathophysiology of hypertension, heart failure and other cardiovascular diseases. It also plays a role in the pathogenesis of "hypertrophy and damage to" The major physiological effects of angiotensin II, such as vasoconstriction, stimulation of aldosterone, regulation of salt and water balance and stimulation of cell growth, are mediated through the type 1 receptor (AT1).
Candesartan cilexetil is a pro-drug for oral use. It is rapidly converted to the active substance, candesartan, by ester hydrolysis during absorption from the gastrointestinal tract. Candesartan is an AIIRA selective for AT1 receptors, with close binding affinity and slow dissociation from the receptor. He has no competitive activity.
Candesartan does not inhibit ACE, which converts angiotensin I to angiotensin II and degrades bradykinin. There is no effect on ACE and no potentiation of bradykinin or substance P. In controlled clinical trials comparing candesartan with ACE inhibitors, the incidence of cough was lower in patients treated with candesartan cilexetil. Candesartan does not bind or block other hormone receptors or ion channels that are important in regulating the cardiovascular system. Angiotensin II receptor (AT1) antagonism results in dose-related increases in plasma renin levels, angiotensin I levels and angiotensin II, and with a decrease in plasma aldosterone concentrations.
Hypertension
In hypertension, candesartan causes a dose-dependent, long-lasting reduction in blood pressure. The antihypertensive action is due to the decrease in peripheral systemic resistance, without reflex increases in heart rate. No severe or exaggerated effects of first dose hypotension or effect were observed "rebound" after stopping treatment.
After administration of a single dose of candesartan cilexetil, the onset of the antihypertensive effect usually occurs within 2 hours. Following continuous treatment, maximum reduction in blood pressure with any dosage is generally achieved within 4 weeks and is maintained during long-term treatment.
According to a meta-analysis, increasing the dose from 16 mg to 32 mg once daily had, on average, a small additional effect. Taking into account the inter-individual variability, a greater effect of the dose may be expected in some patients. average.
Candesartan cilexetil administered once daily causes an effective and homogeneous reduction in blood pressure over 24 hours with a small difference in trough-to-peak ratio during the interval between doses. The antihypertensive effect and tolerability of candesartan and losartan were compared in two randomized double-blind clinical trials involving a total of 1,268 patients with mild to moderate hypertension. The reduction in trough blood pressure (systolic / diastolic) was 13.1 / 10.5 mmHg with candesartan cilexetil 32 mg administered once daily and 10.0 / 8.7 mmHg with losartan potassium 100 mg administered once daily (difference in blood pressure reduction 3.1 / 1.8 mmHg, p
When candesartan cilexetil is combined with hydrochlorothiazide, there is an additive decrease in blood pressure. An increase in the antihypertensive effect is also observed when candesartan cilexetil is used in combination with amlodipine or felodipine.
Medicinal products that block the renin-angiotensin-aldosterone system have a less pronounced antihypertensive effect in black patients (usually low-renin population) than in non-black patients. This also occurs in the case of candesartan. In an open-label clinical study of 5,156 patients with diastolic hypertension, the reduction in blood pressure during treatment with candesartan was significantly less in black patients than in non-blacks (14.4 / 10.3 mmHg vs 19.0 / 12.7 mmHg, p
Candesartan increases renal flow and has no effect or increases glomerular filtration rate, while reducing renal vascular resistance and filtration fraction. In a 3-month clinical study in hypertensive patients with type 2 diabetes mellitus and microalbuminuria, antihypertensive treatment with candesartan cilexetil reduced urinary albumin excretion (mean reduction in albumin / creatinine ratio of 30%, 95% CI 15- 42%). There are currently no data on the effect of candesartan on progression to diabetic nephropathy.
The effects of candesartan cilexetil 8-16 mg (mean dose 12 mg), once daily, on cardiovascular morbidity and mortality were evaluated in a randomized clinical trial in 4,937 elderly patients (age 70-89 years; of which 21% aged 80 years or older) with mild to moderate hypertension followed for a mean of 3.7 years (Study on COgnition and Prognosis in the Elderly). Patients received candesartan cilexetil or placebo with other additional antihypertensive treatments as needed. Blood pressure was reduced from 166/90 to 145/80 mmHg in the candesartan group, and from 167/90 to 149/82 mmHg in the control group. There was no statistically significant difference in the primary end point, major cardiovascular events (cardiovascular mortality, non-fatal stroke and non-fatal myocardial infarction). There were 26.7 events per 1000 patient-years in the candesartan group vs 30.0 events per 1000 patient-years in the control group (relative risk 0.89, 95% CI 0.75 to 1.06, p = 0.19).
Heart failure
Treatment with candesartan cilexetil reduces mortality, reduces hospitalization due to heart failure and improves symptoms in patients with left ventricular systolic dysfunction as demonstrated in the Candesartan in Heart failure - Assessment of Reduction in Mortality and morbidity (CHARM) study.
This double-blind, placebo-controlled study program in patients with chronic heart failure (CHF) NYHA functional class II to IV consisted of three separate studies:
CHARM-alternative (n = 2,028) in patients with left ventricular ejection fraction (LVEF) ≤ 40% not treated with ACE inhibitors due to intolerance (mainly due to cough, 72%), CHARM-added (n = 2,548) in patients with LVEF ≤ 40% and treated with an ACE inhibitor, and CHARM-preserved (n = 3,023) in patients with LVEF> 40%. Patients on optimal background therapy for heart failure (CHF) were randomized to placebo or candesartan cilexetil (titrated from 4 mg or 8 mg once daily up to 32 mg once daily or the highest tolerated dose. , mean dose 24 mg) and followed for a median of 37.7 months. After 6 months of treatment, 63% of patients still taking candesartan cilexetil (89%) had reached the target dose of 32 mg.
In the CHARM-alternative study, the combined endpoint of cardiovascular mortality or first hospitalization for CHF was significantly reduced with candesartan compared to placebo, hazard ratio (HR) 0.77 (95% CI: 0.67 to 0 , 89, cardiovascular or hospitalization for the treatment of heart failure.
The combined end point of all-cause mortality or first hospitalization for CHF was also significantly reduced with candesartan HR 0.80 (95% CI: 0.70 to 0.92, p = 0.001). Of patients treated with candesartan, 36.6% (95% CI: 33.7 to 39.7) and of patients treated with placebo, 42.7% (95% CI: 39.6 to 45.8) had reached this endpoint, absolute difference 6.0% (95% CI: 10.3 to 1.8).
Both mortality and morbidity (hospitalization for CHF), both components of these combined endpoints, contributed to the favorable effects of candesartan. Treatment with candesartan cilexetil resulted in an improvement in NYHA functional class (p = 0.008).
In the CHARM-added study, the combined endpoint of cardiovascular mortality or first hospitalization for CHF was significantly reduced with candesartan compared to placebo HR 0.85 (95% CI: 0.75 to 0.96, p = 0.011) This corresponds to a relative risk reduction of 15%. Of candesartan-treated patients, 37.9% (95% CI: 35.2 to 40.6) and of placebo-treated patients, 42.3% (95 % CI: 39.6 to 45.1) met this endpoint, absolute difference 4.4% (95% CI: 8.2 to 0.6). 23 patients had to be treated for the duration of the study to prevent death from cardiovascular events or hospitalization for treatment of heart failure in a patient. The combined end point of all-cause mortality or first hospitalization for CHF was also significantly reduced with candesartan HR 0.87, (95% CI: 0.78 to 0.98, p = 0.021). candesartan-treated patients, 42.2% (95% CI: 39.5 to 45.0) and of placebo-treated patients, 46.1% (95% CI: 43.4 to 48.9) achieved this endpoint, absolute difference 3.9% (95% CI: 7.8 to 0.1) Both mortality and morbidity, both components of these combined endpoints, contributed to the favorable effects of candesartan. Treatment with candesartan cilexetil resulted in an improvement in NYHA functional class (p = 0.020).
In the CHARM-preserved study, a statistically significant reduction in the combined end-point of cardiovascular mortality or first hospitalization for CHF, HR 0.89, was not obtained (95% CI: 0.77 to 1.03, p = 0.118).
All cause mortality was not statistically significant when examined separately for each of the three CHARM studies. However, all cause mortality was also assessed in pooled populations, in the CHARM-alternative and CHARM-added studies, HR 0.88, (95% CI: 0.79 to 0.98, p = 0.018) and in all three studies, HR 0.91 (95% CI: 0.83 to 1.00, p = 0.55).
The beneficial effects of candesartan were consistent regardless of age, gender and concomitant medications. Candesartan was also effective in patients taking both beta-blockers and ACE inhibitors at the same time, and the benefit was obtained whether or not patients took ACE inhibitors at the target dose recommended by the treatment guidelines.
In patients with CHF and impaired left ventricular systolic function (left ventricular ejection fraction, LVEF ≤40%), candesartan decreases systemic vascular resistance and pulmonary capillary wedge pressure, increases plasma renin activity and blood concentration. angiotensin II, and reduces aldosterone levels.
05.2 "Pharmacokinetic properties -
Absorption and distribution
Following oral administration, candesartan cilexetil is converted to the active substance candesartan. The absolute bioavailability of candesartan is approximately 40% after administration of an oral solution of candesartan cilexetil. The relative bioavailability of the tablet formulation compared to the same oral solution is approximately 34% with very little variability. The estimated absolute bioavailability of the tablet is therefore 14%. Mean peak concentration values (Cmax) are reached within 3-4 hours after dosing. Serum concentrations of candesartan increase linearly with increasing doses in the therapeutic range. No differences in candesartan pharmacokinetics were observed in either sex. The area under the curve (AUC) is not significantly affected by food.
Candesartan is highly bound to plasma proteins (more than 99%). The apparent volume of distribution of candesartan is 0.1 L / kg.
The bioavailability of candesartan is not affected by food.
Biotransformation and elimination
Candesartan is eliminated almost entirely unchanged via the urinary and biliary routes and only to a lesser extent via hepatic metabolism (CYP2C9). Available interaction studies indicate no effect on CYP2C9 and CYP3A4. Based on data in vitro, no interaction is expected to occur in vivo with drugs whose metabolism depends on cytochrome P450, CYP1A2, CYP2A6, CYP2C9, CYP2C19, CYP2D6, CYP2E1 or CYP3A4 isoenzymes. The terminal half-life is approximately 9 hours. No accumulation is observed following repeated dosing.
Total plasma clearance of candesartan is approximately 0.37 mL / min / kg, with a renal clearance of approximately 0.19 mL / min / kg. Renal excretion occurs by both glomerular filtration and active tubular secretion. Following an oral dose of 14C-labeled candesartan cilexetil, approximately 26% of the dose is excreted in the urine as candesartan and 7% as an inactive metabolite, while approximately 56% of the dose is found in the faeces as candesartan and 10% as the inactive metabolite.
Pharmacokinetics in special populations
In the elderly (over 65 years of age) both Cmax and AUC of candesartan are increased by approximately 50% and 80%, respectively, compared to young subjects. However, the blood pressure response and the incidence of adverse events are similar after administration of the same dose of Atacand in young and elderly patients (see section 4.2).
In patients with mild and moderate renal impairment, Cmax and AUC of candesartan during repeated administration increased by approximately 50% and 70%, respectively, but t½ was not altered compared to patients with normal renal function. Corresponding changes in patients with severe renal insufficiency were approximately 50% and 110%. The terminal t½ of candesartan was approximately doubled in patients with severe renal impairment. The AUC of candesartan in hemodialysis patients was similar to that in patients with severe renal impairment.
In two studies, both in patients with mild to moderate hepatic impairment, an increase in mean AUC of Candesartan of approximately 20% in one study and 80% in the other study was observed (see section 4.2). experience in patients with severe hepatic impairment.
05.3 Preclinical safety data -
No abnormal systemic or target organ toxicity was observed at clinically relevant doses. In preclinical safety studies candesartan had effects on kidney and red cell parameters at high doses in mice, rats, dogs and monkeys. Candesartan caused a reduction in red blood cell parameters (erythrocytes, hemoglobin, hematocrit). Effects on the kidneys (such as interstitial nephritis, tubular distension, tubular basophilia; increased plasma concentrations of BUN and creatinine) were induced by candesartan and may be secondary to the hypotensive effect resulting in impaired renal perfusion. Furthermore, candesartan induced hyperplasia / hypertrophy of juxtaglomerular cells These changes were considered to be caused by the pharmacological action of candesartan. With therapeutic doses of candesartan in humans, hyperplasia / hypertrophy of the juxtaglomerular cells does not appear to have any relevance.
Foetotoxicity has been observed in late pregnancy (see section 4.6).
The data of mutagenesis in vitro and in vivo indicate that candesartan does not exert mutagenic or clastogenic activity under conditions of clinical use.
No carcinogenic phenomena were observed.
06.0 PHARMACEUTICAL INFORMATION -
06.1 Excipients -
Calcium Carmellose
Hydroxypropylcellulose
Iron Oxide, CI 77491 (E172) (for 8 mg, 16 mg and 32 mg tablets only)
Lactose monohydrate
Magnesium stearate
Cornstarch
Macrogol
06.2 Incompatibility "-
Not relevant.
06.3 Period of validity "-
3 years.
06.4 Special precautions for storage -
This medicinal product does not require any special storage temperatures.
06.5 Nature of the immediate packaging and contents of the package -
PVC / PVDC blisters of 7, 14, 15, 15x1 (single dose), 20, 28, 30, 30x1 (single dose), 50, 50x1 (single dose), 56, 90, 98, 98x1 (single dose), 100 and 300 tablets.
HDPE bottles containing 100 and 250 tablets
Not all pack sizes may be marketed.
06.6 Instructions for use and handling -
No special instructions.
07.0 HOLDER OF THE "MARKETING AUTHORIZATION" -
AstraZeneca S.p.A. - Palazzo Ferraris, via Ludovico il Moro 6 / C - Basiglio (MI) 20080
08.0 MARKETING AUTHORIZATION NUMBER -
Blister packs :
4 mg tablets - 7 tablets in PVC / PVDC blister - AIC n. 033577038
4 mg tablets - 14 tablets in PVC / PVDC blister - AIC n. 033577040
4 mg tablets - 15 tablets in PVC / PVDC blister - AIC n. 033577584
4 mg tablets - 15x1 tablets in PVC / PVDC blister - AIC n. 033577634
4 mg tablets - 20 tablets in PVC / PVDC blister - AIC n. 033577053
4 mg tablets - 28 tablets in PVC / PVDC blister - AIC n. 033577065
4 mg tablets - 30 tablets in PVC / PVDC blister - AIC n. 033577572
4 mg tablets - 30x1 tablets in PVC / PVDC blister - AIC n. 033577646
4 mg tablets - 50 tablets in PVC / PVDC blister - AIC n. 033577077
4 mg tablets - 50x1 tablets in PVC / PVDC blister - AIC n. 033577394
4 mg tablets - 56 tablets in PVC / PVDC blister - AIC n. 033577089
4 mg tablets - 90 tablets in PVC / PVDC blister - AIC n. 033577711
4 mg tablets - 98 tablets in PVC / PVDC blister - AIC n. 033577091
4 mg tablets - 98x1 tablets in PVC / PVDC blister - AIC n. 033577103
4 mg tablets - 100 tablets in PVC / PVDC blister - AIC n. 033577115
4 mg tablets - 300 tablets in PVC / PVDC blister - AIC n. 033577127
Bottle packs :
4 mg tablets - 100 tablets in HDPE-AIC bottle n. 033577139
4 mg tablets - 250 tablets in HDPE bottle - AIC n. 033577141
Blister packs :
8 mg tablets - 7 tablets in PVC / PVDC blister - AIC n. 033577154
8 mg tablets - 14 tablets in PVC / PVDC blister - AIC n. 033577166
8 mg tablets - 15 tablets in PVC / PVDC blister - AIC n. 033577608
8 mg tablets - 15x1 tablets in PVC / PVDC blister - AIC n. 033577659
8 mg tablets - 20 tablets in PVC / PVDC blister - AIC n. 033577178
8 mg tablets - 28 tablets in PVC / PVDC blister - AIC n. 033577180
8 mg tablets - 30 tablets in PVC / PVDC blister - AIC n. 033577596
8 mg tablets - 30x1 tablets in PVC / PVDC blister - AIC n. 033577661
8 mg tablets - 50 tablets in PVC / PVDC blister - AIC n. 033577192
8 mg tablets - 50x1 tablets in PVC / PVDC blister - AIC n. 033577406
8 mg tablets - 56 tablets in PVC / PVDC blister - AIC n. 033577204
8 mg tablets - 90 tablets in PVC / PVDC blister - AIC n. 033577723
8 mg tablets - 98 tablets in PVC / PVDC blister - AIC n. 033577216
8 mg tablets - 98x1 tablets in PVC / PVDC blister - AIC n. 033577228
8 mg tablets - 100 tablets in PVC / PVDC blister - AIC n. 033577230
8 mg tablets - 300 tablets in PVC / PVDC blister - AIC n. 033577242
Bottle packs :
8 mg tablets - 100 tablets in HDPE bottle - AIC n. 033577255
8 mg tablets - 250 tablets in HDPE bottle - AIC n. 033577267
Blister packs :
16 mg tablets - 7 tablets in PVC / PVDC blister - AIC n. 033577279
16 mg tablets - 14 tablets in PVC / PVDC blister - AIC n. 033577281
16 mg tablets - 15 tablets in PVC / PVDC blister - AIC n. 033577622
16 mg tablets - 15x1 tablets in PVC / PVDC blister - AIC n. 033577673
16 mg tablets - 20 tablets in PVC / PVDC blister - AIC n. 033577293
16 mg tablets - 28 tablets in PVC / PVDC blister - AIC n. 033577305
16 mg tablets - 30 tablets in PVC / PVDC blister - AIC n. 033577610
16 mg tablets - 30x1 tablets in PVC / PVDC blister - AIC n. 033577685
16 mg tablets - 50 tablets in PVC / PVDC blister - AIC n. 033577317
16 mg tablets - 50x1 tablets in PVC / PVDC blister - AIC n. 033577418
16 mg tablets - 56 tablets in PVC / PVDC blister - AIC n. 033577329
16 mg tablets - 90 tablets in PVC / PVDC blister - AIC n. 033577735
16 mg tablets - 98 tablets in PVC / PVDC blister - AIC n. 033577331
16 mg tablets - 98x1 tablets in PVC / PVDC blister - AIC n. 033577343
16 mg tablets - 100 tablets in PVC / PVDC blister - AIC n. 033577356
16 mg tablets - 300 tablets in PVC / PVDC blister - AIC n. 033577368
Bottle packs :
16 mg tablets - 100 tablets in HDPE bottle - AIC n. 033577370
16 mg tablets - 250 tablets in HDPE bottle - AIC n. 033577382
Blister packs :
32 mg tablets - 7 tablets in PVC / PVDC blister - AIC n. 033577420
32 mg tablets - 14 tablets in PVC / PVDC blister - AIC n. 033577432
32 mg tablets - 15 tablets in PVC / PVDC blister - AIC n. 033577444
32 mg tablets - 15x1 tablets in PVC / PVDC blister - AIC n. 033577697
32 mg tablets - 20 tablets in PVC / PVDC blister - AIC n. 033577457
32 mg tablets - 28 tablets in PVC / PVDC blister - AIC n. 033577469
32 mg tablets - 30 tablets in PVC / PVDC blister - AIC n. 033577471
32 mg tablets - 30x1 tablets in PVC / PVDC blister - AIC n. 033577709
32 mg tablets - 50 tablets in PVC / PVDC blister - AIC n. 033577483
32 mg tablets - 50x1 tablets in PVC / PVDC blister - AIC n. 033577495
32 mg tablets - 56 tablets in PVC / PVDC blister - AIC n. 033577507
32 mg tablets - 90 tablets in PVC / PVDC blister - AIC n. 033577747
32 mg tablets - 98 tablets - in PVC / PVDC AIC blister n. 033577519
32 mg tablets - 98x1 tablets in PVC / PVDC blister - AIC n. 033577521
32 mg tablets - 100 tablets in PVC / PVDC blister - AIC n. 033577533
32 mg tablets - 300 tablets in PVC / PVDC blister - AIC n. 033577545
Bottle packs :
32 mg tablets - 100 tablets in HDPE bottle - AIC n. 033577558
32 mg tablets - 250 tablets in HDPE bottle - AIC n. 033577560
09.0 DATE OF FIRST AUTHORIZATION OR RENEWAL OF THE AUTHORIZATION -
4 mg
17 December 1997/28 April 2012
8 mg- 16 mg- 32 mg
22 December 2005/28 April 2012
10.0 DATE OF REVISION OF THE TEXT -
09/2015