Lactic acid (C3H6O3) is a substance produced by the body during normal body metabolism. This synthesis becomes particularly intense in conditions of lack of oxygen, that is when the metabolic demand of this gas exceeds the availability; it is a characteristic juncture of "strenuous physical exercise, but also of particular pathological states, such as those resulting from a" airway obstruction.
Biochemical basis
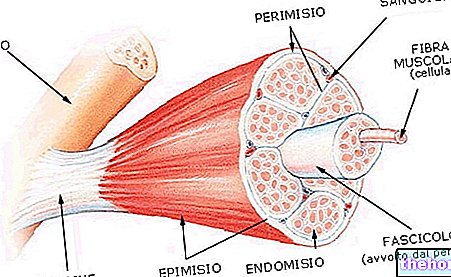
Let us briefly recall that "lactic acid is produced starting from pyruvate, which represents the final product of glycolysis (cytoplasmic process that results in the degradation of glucose into two molecules of pyruvic acid or pyruvate). In the sixth of the ten stages of glycolysis, the" 3-phosphoglycerine aldehyde is oxidized thanks to oxidized NAD (NAD +) which acts as an acceptor of H + hydrogen ions. The NAD is then reduced to NADH (H +). At this point, if we want the energy to continue to be generated through glycolysis, we must take care of regenerating the oxidized NAD (NAD +), which otherwise would be rapidly depleted until it is exhausted. When the availability of oxygen is sufficient, the reoxidation of the reduced NAD is entrusted to the Krebs cycle (mitochondrial oxidative phosphorylation), with oxygen consumption, water formation and ATP synthesis. When oxygen is scarce, the pyruvate that does not enter the krebs cycle is reduced to lactic acid by the enzyme lactate dehydrogenase From this reaction (see figure), the NAD + necessary for the further reaction of the 3-phosphoglycerine aldehyde is restored; glycolysis can then proceed.

Once produced, at physiological pH, lactic acid tends to dissociate almost entirely into two ions: the lactate ion and the H + ion (according to the reaction shown in the figure).
Being, as the name itself reminds us, of an acid, the excessive production of lactate and H + tends to lower the pH inside the cell, contributing (together with many other factors) to the onset of fatigue.
The first mechanism implemented by the cells to defend themselves from the excessive production of lactic acid consists in its efflux towards the extracellular environment and the blood. Not surprisingly, in normal conditions the blood lactate concentration is equal to 1-2 mmol / L, while it rises up to over 20 mmol / L during particularly intense physical exercise.
Disposal of lactic acid
Although at high concentrations lactic acid is a particularly toxic product, which as such must necessarily be disposed of, it cannot and must not be considered a waste. On the contrary, once produced, lactic acid can:
- be captured and used by some tissues for energy purposes, such as in the heart (which prefers to use lactate rather than glucose), but also at the level of the muscle cells themselves (white fibers are better at producing it and red ones at disposing of it) ;
- be used for the ex-novo synthesis of glucose / glycogen (gluconeogenesis, Cori cycle in the liver).
In both cases, the lactate must first of all be reconverted into pyruvate, again by the enzyme lactate-dehydrogenase, with a reduction of NAD + to NADH (H +). At this point, the pyruvate can be completely oxidized in the Krebs cycle or be used for gluconeogenesis.
We have already seen how an excessive synthesis of lactic acid disturbs the metabolism of the cell, which releases it externally through specific membrane transporters (MCT). In addition to various defense mechanisms that we will see shortly, there is a priori a further control that prevents the excessive accumulation of lactate in the intracellular environment. The drop in pH (acid environment) - due to the accumulation of H + hydrogen ions deriving from the dissociation of lactic acid - inhibits the enzyme phosphofructokinase, which intervenes in the third stage of glycolysis determining its speed. Consequently, an excessive drop in pH causes a slowdown in glycolysis, reducing the rate of synthesis of lactic acid (negative feedback).
The excessive decrease of the intracellular pH is however also fought by the buffer systems, among which the most important is the biarbonate / carbonic acid one, enhanced by the respiratory activity with the elimination of CO2:



As shown in the figure, the intense respiratory activity that occurs during intense physical exercise reduces the concentration of CO2 and carbonic acid in the blood, buffering the intake of the H + produced by dissociation of lactic acid.

The image above shows the temporal course of blood lactate (lactataemia) during the recovery phase following an intense lactacid effort. As clearly shown by the graph, the trained subject is able to dispose of lactic acid in a shorter time than the sedentary one. Another important thing to underline is that within one hour, at most, the levels of milk temperature return to the same conditions. basal; therefore it is wrong to attribute to the accumulation of lactic acid the muscle soreness that accompanies the days following a particularly intense workout.
To facilitate the elimination of lactic acid after a maximum effort, the athlete will take care to follow the performance with a cool-down phase at a light pace lasting 15-20 minutes.